● INTRODUCTION
The spectrum of congenital cardiac anomalies presented in this book can be better understood if the reader has some basic knowledge on cardiac development. In the last 30 years, knowledge on the embryology of the human heart has experienced significant change, mainly due to advances in molecular genetics and lineage tracing studies (1–6). This acquired knowledge has shed new light on the origin of different cardiac compartments in the embryonic heart and the undergoing cell differentiation, suggesting that the primary heart tube is similar to a scaffold, where cells from various surrounding cell lines are added during cardiac development (5, 6). This knowledge provides a basis for a better understanding of the pathogenesis of congenital heart malformations (3). In this chapter, the classic steps of cardiac morphogenesis will be presented with the understanding that in this traditional concept of cardiac and large vessel embryogenesis, many questions remain unanswered (2). This chapter will also present cardiac morphogenesis along the model proposed by recent theories of the past two decades. Associated gene expression of cardiac embryogenesis and the development of the cardiac conduction system are not presented given the scope of this book. For more detailed information, we recommend recent monographs and review articles on cardiac embryology (1–6).
● TRADITIONAL APPROACH TO EMBRYOLOGY OF THE HUMAN HEART
In the third-week postconception, the embryo consists of three basic germ layers: the ectoderm, the mesoderm, and the endoderm. The mesoderm differentiates into four compartments: axial, paraxial, intermediate, and lateral. The lateral mesoderm is involved in the formation of the circulatory system and viscera. In this lateral splanchnic mesoderm, clusters of angiogenic cardiac precursor cells develop and migrate anteriorly toward the midline and fuse into a single heart tube. These bilateral crescent-like cardiac plates are asymmetric and determine the rotation of the heart (2).
The classic teaching of embryonic heart development focused on the following major steps:
Step 1: Primitive Heart Tube Formation: In the cardiogenic plate islands of progenitor, cells develop as paired cells and fuse to form the midline primitive heart tube (Fig. 3.1) (4). The primitive heart tube is anchored caudally by the vitelloumbilical veins and cranially by the dorsal aortae and pharyngeal arches. The primitive heart tube shows folding zones or transitional zones with the most prominent being the primary fold (PF) at the arterial pole and the atrioventricular ring (AVR) at the venous pole (Fig. 3.2). These transitional zones will later form the cardiac septa and valves.
Step 2: Heart Tube Looping: The primitive heart tube shows peristaltic movements and with growth the tube undergoes looping by folding on itself and to the right and anterior, resulting in future atria, ventricles, and outflow tracts (Figs. 3.2 to 3.4) (4). The process of looping begins with bulging of the tube and looping rightward with the primitive ventricle moving downward to the right while the primitive atrium moving upward and to the left behind the ventricle (Figs. 3.3 and 3.4). This asymmetric looping direction is possibly established by the clockwise rotation of cilia. At this stage, waves of peristalsis are recognized within the heart tube and tube pulsations are first recognized around day 21 to 22 postconception (day 35–36 menstrual age, end 5th gestational week). Various regions can be recognized in the folded tube to include (Figs. 3.3 and 3.4) the sinus venosus at the venous pole, the sinoatrial ring (SAR), the primitive atrium, the atrioventricular ring encircling the future atrioventricular canal, the primitive left ventricle (LV), the primary fold or ring which becomes the interventricular septum, the primitive right ventricle (RV), the outflow tract or common trunk ending at the ventriculoarterial ring (VAR), and the aortic sac (AS) at the arterial end (Fig. 3.4).
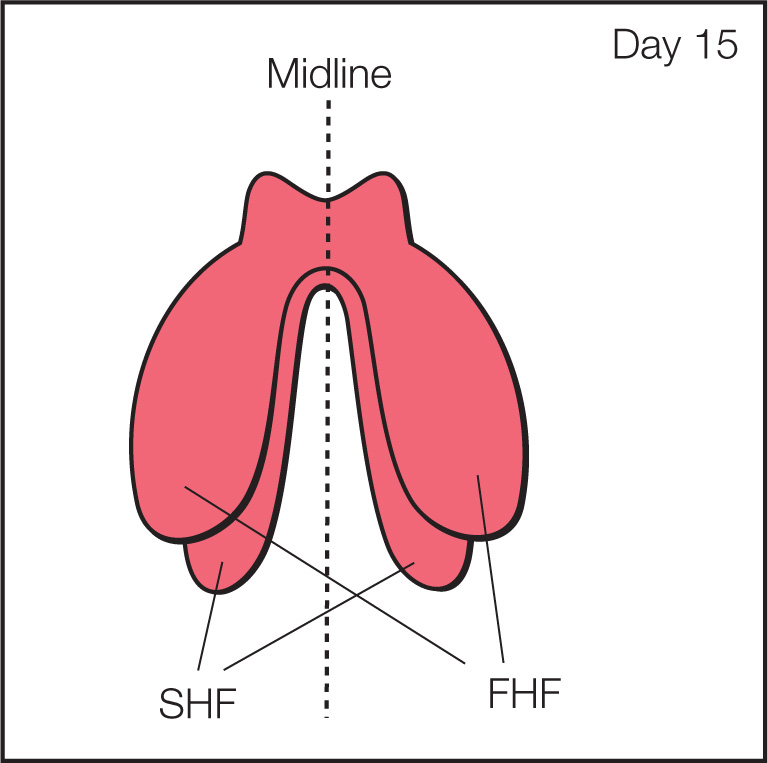
Figure 3.1: Frontal view of the cardiac crescent stage of cardiac morphogenesis. In the primitive plate, bilateral fields of cardiac mesoderm are present. Part of the mesoderm layer gives rise to the cardiogenic fields, located on both sides of the midline (dashed line). The cardiogenic fields consist of a first (FHF) and second (SHF) heart fields. The two sides of the cardiac crescent fuse along the midline to form the primitive heart tube (see Fig. 3.2).
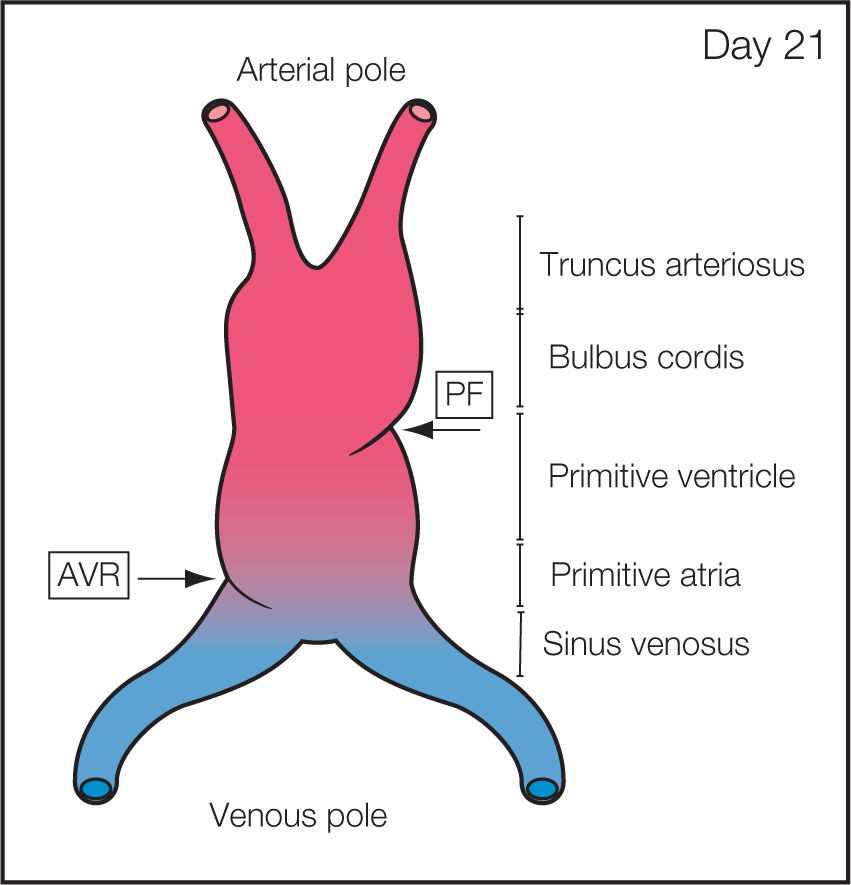
Figure 3.2: Frontal view of the primitive heart tube stage of cardiac morphogenesis. Once the primitive heart tube is formed, a primitive venous pole on one end and an arterial pole on the other end are seen. Primitive regions along the heart tube include from caudal to rostral: sinus venosus, primitive atria, primitive ventricle, bulbus cordis (conus), and truncus arteriosus. The regions are separated by so-called transitional zones, which will later form the septa and valves. Two transitional zones are identifiable in this schematic: the atrioventricular ring (AVR) forming the future atrioventricular valves and the primary fold (PF) forming the future interventricular septum.
Step 3: Atria, Ventricles, and Outflow Tracts Septation: Within this tube and at different sites, septations occur to differentiate the two atria (Fig. 3.5), two ventricles, two atrioventricular valves, and two separate outflow tracts. The paired branchial arteries with two aortae progressively regress, resulting in a left aortic arch with its corresponding bifurcations. On the venous side, different paired veins regress and fuse to develop the systemic venous system with the hepatic veins and superior and inferior venae cavae (2). The following sections provide details of the septation process.
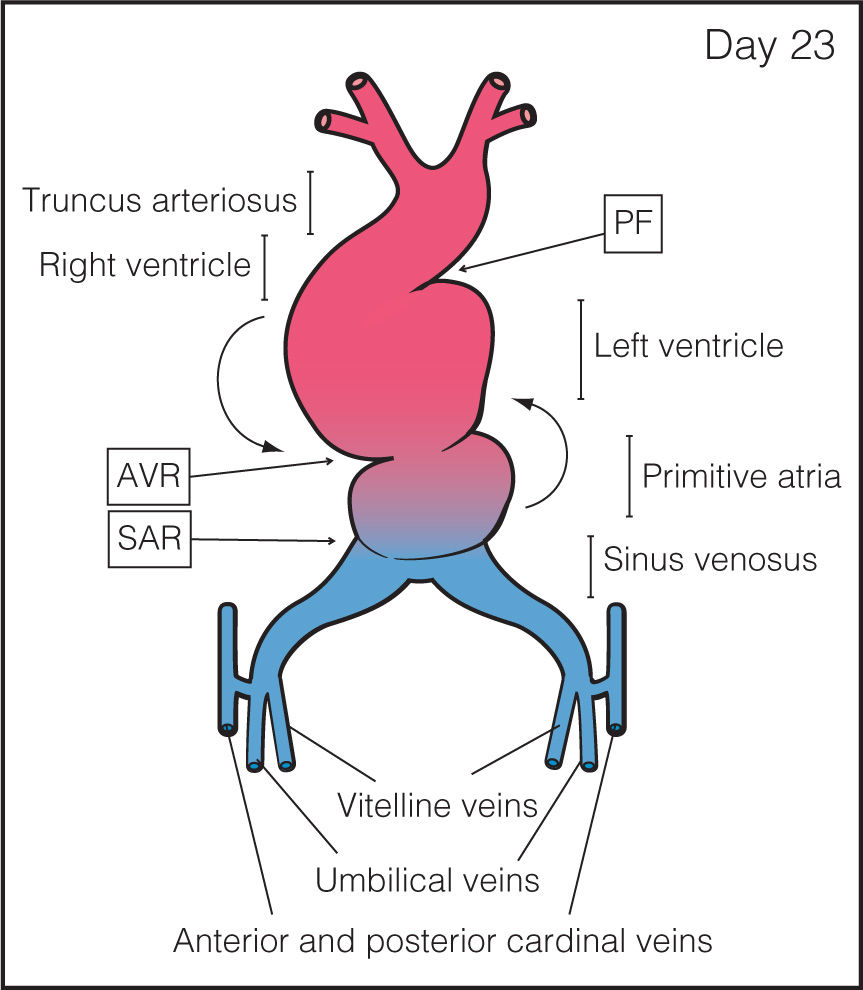
Figure 3.3: Frontal view of the heart tube during the cardiac looping stage of cardiac morphogenesis. The heart tube starts to grow and contract in a pulsatile manner. At this stage, the cardiac tube starts to loop with folding along the long axis and rotation to the right and ventral, resulting in a D-looped heart. During this looping, the primitive ventricle is pushed downward and to the right while the atria move upward and posterior to the ventricles (curved arrows). As the tubular heart elongates, it bends on itself, forming an S-shaped heart. Primitive cardiac chambers are better identified and are separated by transitional zones as the sinoatrial ring (SAR), the atrioventricular ring (AVR), and the primary fold (PF).
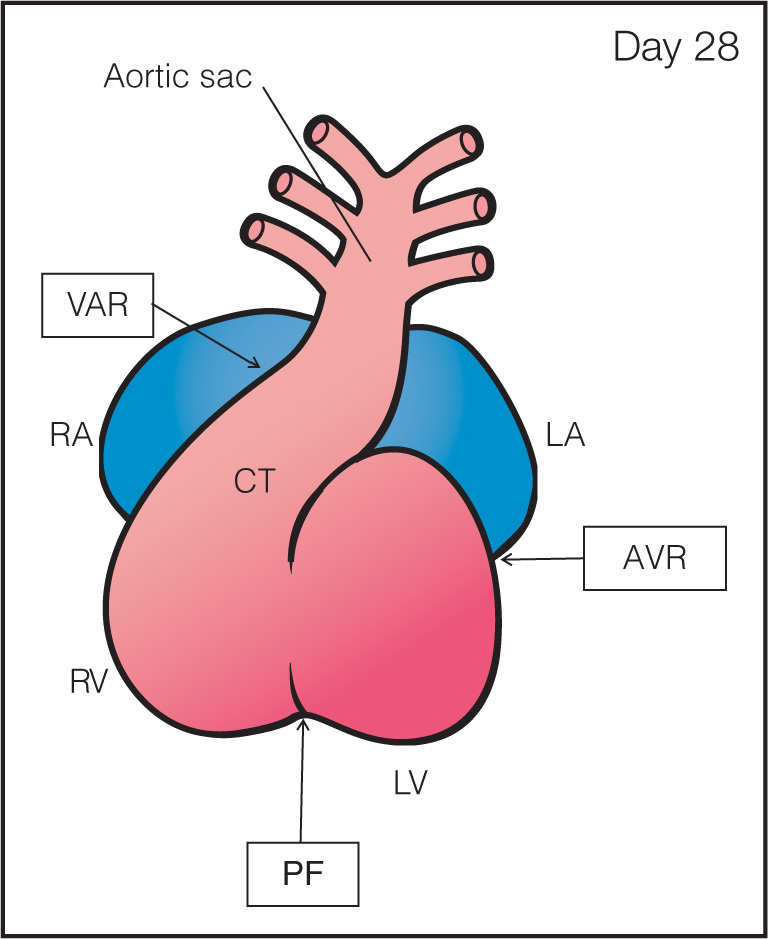
Figure 3.4: Frontal view of the looped heart (closed) during the septation of atria, ventricles, and great vessels (see Fig. 3.6) for open heart. After looping, several transitional zones can be identified separating the primitive cardiac chambers (compare with Fig. 3.6). The four transitional zones or rings are the sinoatrial ring in between the sinus venosus and common atrium (not seen here, see Fig. 3.6), the atrioventricular ring (AVR) between the common atrium and common ventricle, the primary fold (PF) between the primitive left (LV) and right (RV) ventricles, and the ventriculoarterial ring (VAR) in the conotruncus (CT) region of the outflow tract of the heart. At this stage, the septation of atria, ventricles, and great vessels occurs. RA, right atrium; LA, left atrium.
Septation of the Atria
The primitive atrium is divided into two by the formation of two septa: the septum primum and the septum secundum. The first septum to develop is the septum primum and it forms by descending from the roof of the common atrium in the direction of the endocardial cushions (Fig. 3.5A). During the ingrowth of this septum, a communication between both atria remains open and is called the foramen primum. During this development, the septum primum does not completely close the interatrial communication since fenestrations occur in its center, forming a second communication, called the foramen secundum (Fig. 3.5B). The second septum, septum secundum, develops in a crescent shape to the right side of the septum primum and grows from ventral to dorsal. The septum secundum remains incomplete and almost covers the free rim of the septum primum (Fig. 3.5B). Within the developing septum secundum, an ovale-shaped orifice is formed, which is the foramen ovale or foramen secundum (Fig. 3.5C). Both septa fuse except for the foramen ovale region, which remains patent for shunting blood from right to left atrium (Fig. 3.5D,E). The free flap of the septum primum is seen on ultrasound within the left atrium as the foramen ovale flap. Septation of the atrium occurs between day 45 and 60 postconception and completion of this process occurs after birth with the closure of the foramen ovale.
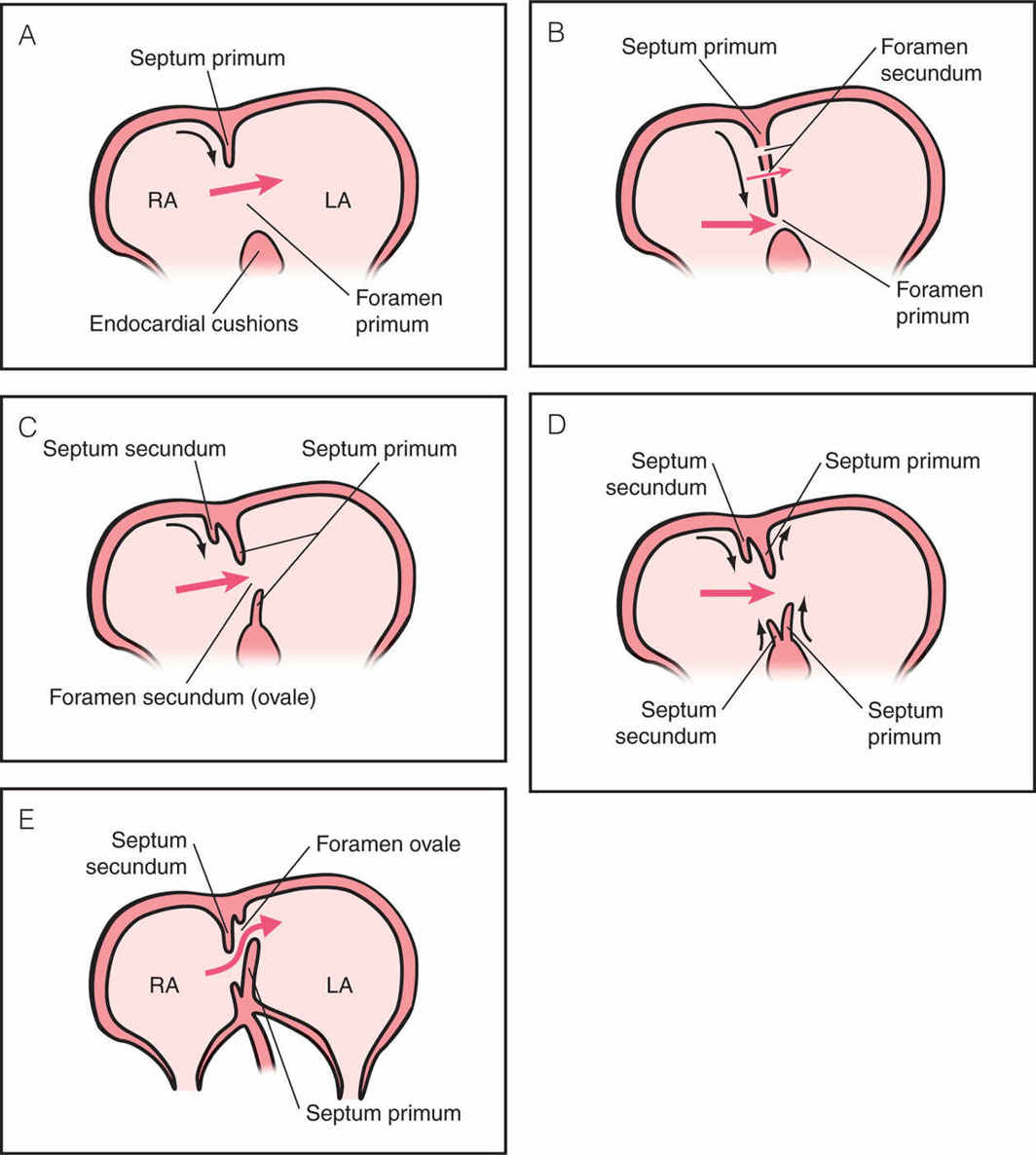
Figure 3.5: Stages (A–E) of atrial septation into right (RA) and left (LA) atria with formation of septum primum and secundum. A: A wide communication, called foramen primum, is seen between the primitive RA and LA. The septum primum grows from the atrial roof toward the endocardial cushions (curved arrow). Red arrow shows direction of blood flow. B: The septum primum continues its growth (curved arrow) and is seen to almost completely close the foramen primum. In the middle of the septum primum, perforations appear to allow blood flow from right to left atrium. This new communication is the foramen secundum, the future foramen ovale. C: Foramen primum is now closed together with the formation of the atrioventricular valves (not shown here). From now until birth the foramen secundum or ovale is the main interatrial communication. The septum secundum grows from the atrial roof along the septum primum (curved arrow) to partly cover the foramen ovale. D: The septum secundum continues its growth (curved arrows) from both directions with an upper and a lower limb. The lower limb is also called dorsal mesenchymal protrusion. The lower limb of the septum primum continues its growth while the upper limb begins to regress. The boundaries of the foramen ovale are thus formed by the upper limb of the septum secundum and the lower limb of the septum primum (see E). E: At this stage, the crux of the embryonic heart has developed into its final shape in fetal life with two separate atrioventricular valves and a closed interventricular septum. The interatrial septum is now mainly formed by the septum secundum with the exception of the opening of the foramen ovale as the interatrial communication. To the left side of the septum secundum, the upper limb of the septum primum has regressed while the lower limb forms the flap or the valve of the foramen ovale, which will close after birth. This construct allows during fetal life oxygenated blood from the ductus venosus, to be preferentially directed into the left atrium (curved red arrow), left ventricle, ascending aorta toward the coronary and brain circulations (left pathway). The majority of deoxygenated blood from the superior and inferior venae cavae and coronary sinus is directed into the right atrium to reach the right ventricle, pulmonary artery, ductus arteriosus, descending aorta, and then to the placenta to be reoxygenated (right pathway).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


