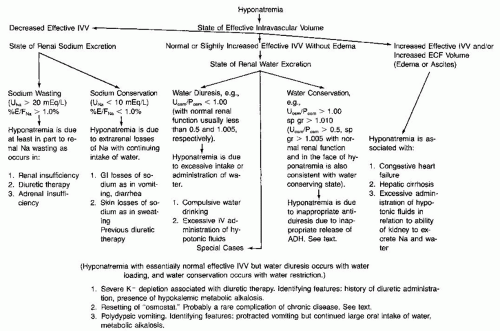
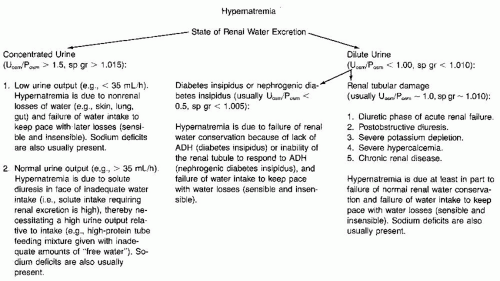
CLINICAL ASSESSMENT OF DISORDERS OF WATER AND ELECTROLYTE METABOLISM
Disorders of extracellular fluid (ECF) electrolyte composition may be detected by measurement of the serum electrolyte concentrations. Identification of the process (or processes) behind the disturbance of electrolyte composition and the planning of subsequent therapy are critically dependent on the clinician’s ability to accurately assess whether the disturbance in ECF electrolyte composition is associated with volume expansion, volume contraction, or a normal volume.
In the evaluation of a patient’s ECF volume status, it must be kept clearly in mind that the critical volume that is effective in determining cardiac output is the effective intravascular volume (IVV). The most effective IVV is that which maintains an optimal cardiac output and thus maximizes tissue perfusion. Although the actual IVV and the effective IVV are the same in many clinical situations and can be expected to change in direct proportion, in a number of important clinical states, the actual IVV is different from the effective IVV. For example, in acute metabolic acidosis, increased venoconstriction can develop, resulting in an abnormal increase in central venous pressure (CVP) and cardiac output. Under this circumstance, the actual IVV could be less than normal, whereas the effective IVV is greater than normal. Because of the increase in venous tone, an IVV that is lower than normal can maintain a normal effective IVV.
Acute changes in venous tone induced by drugs (e.g., morphine, furosemide, norepinephrine), changes in acid-base status, and the presence of bacterial endotoxin also can disrupt the normal relation between the actual IVV and the effective IVV.
The most reliable clinical means for assessing the status of the effective IVV is the pulmonary capillary wedge pressure. This measurement is an estimate of the pulmonary capillary pressure, which is a measure of the filling pressure of the left ventricle. Factors that increase pulmonary capillary wedge pressure tend to increase cardiac output by increasing capillary outflux. When effective IVV is considered within these constraints, it becomes clear that under virtually any physiologic or pathophysiologic circumstance, an optimal effective IVV is one that results in a pulmonary capillary wedge pressure that is high enough to promote optimal cardiac output but low enough to prevent pulmonary edema.
Fortunately, in most clinical situations, it is not necessary to resort to measuring pulmonary wedge pressure to assess whether a disturbance of ECF composition is associated with an effective IVV that is abnormally high, abnormally low, or normal. Instead, an accurate assessment of the effective IVV usually can be made by a careful clinical assessment using the criteria listed in
Table 10.1. This table lists the bedside and laboratory means to assess volume status according to whether the findings are consistent with an effective IVV that is less than normal or an effective IVV that is nearly normal or expanded.
Also shown in
Table 10.1 are the conditions under which the given means for evaluating the IVV must be qualified (i.e., the conditions that may render the meaning of the finding indeterminate with respect to the evaluation of IVV). For example, the relation between an increase in weight and a change in IVV is rendered indeterminate if, at the same time, the patient has developed a third space, as in bowel obstruction. In this instance, the entire weight gain could be caused by the accumulation of fluid outside the IVV. Thus, the finding of weight gain in this setting cannot be used as evidence of an increase in effective IVV. Whenever a finding can be significantly qualified,
it should not be used in the assessment of the effective IVV. As many independent means as practical should be used to assess the effective IVV to minimize the effect of possible error on the final decision. The greater the number of independent, unqualified findings that agree in favor of a given clinical decision, the more likely it is that the decision is correct. If such a systematic approach to clinical decision making is used, it should be possible to arrive at an accurate evaluation of volume status in most circumstances.
DATABASE FOR ASSESSMENT OF EFFECTIVE INTRAVASCULAR VOLUME
Body Weight
All patients should be weighed on admission to the hospital and then periodically during their hospital stay. In patients undergoing surgery, or in whom problems in fluid and electrolyte balance are anticipated, weight must be measured daily.
Alterations in body weight are the result of changes in body water content plus solid tissue content (fat, protein, bone). Gains or losses of solid tissue are almost always related to changes in caloric intake and seldom exceed 0.25 kg/24 hours. For example, a patient who takes no calories for 24 hours is forced to consume her endogenous stores of fat and protein to meet the energy requirements for continued life. The complete oxidation of fat yields 9 cal/g, and protein yields 4 cal/g. It can be readily calculated that the complete oxidation of 0.25 kg of solid tissue (in starvation, a mixture of about 87% fat, 13% protein) yields enough calories to meet basal daily energy needs. Thus, changes in weight exceeding 0.25 kg/24 hours are almost always attributable to changes in water balance. Although the relation between body weight and effective IVV can be variable, usually the relation between changes in body weight and IVV can be correctly assessed by the application of the guidelines. The first is that a decrease in body weight below normal (for the patient), and not explained on the basis of inadequate caloric intake, can be assumed
to be accompanied by a decrease in IVV. The second is that an increase in body weight above normal not explained by increased nutrition can be assumed to be accompanied by an increase in IVV except when the weight gain develops in association with the following conditions:
Significant hypoalbuminemia: serum albumin less than 2.5 g/dL
Venous obstruction or congestion
Development of third spaces (e.g., obstructed or ischemic bowel)
Under these three general conditions, an increase in body weight may not reflect an increase in the effective IVV.
Renal Function
Creatinine, a by-product of muscle energy metabolism, is produced at a constant rate that is related to muscle mass. Nearly all of the creatinine produced is excreted by glomerular filtration. Therefore, changes in the concentration of serum creatinine reflect changes in the glomerular filtration rate (GFR), and the clearance of creatinine is an index of the GFR.
Normally, as muscle mass increases, the GFR increases proportionately less. Therefore, on the average, children have lower serum creatinine values than do adults, and large adults have higher serum creatinine levels than do small adults. Because of these considerations, a single range of serum creatinine values cannot be applied to everyone.
The following guidelines are suggested for the evaluation of the IVV in light of the state of renal function. Azotemia can be assumed to result from decreased renal perfusion if the serum creatinine level is elevated, the urine is concentrated (specific gravity higher than 1.015), and renal sodium conservation is present (urine sodium level <20 mEq/L) on a random and untimed urine sample. If the fractional excretion of sodium is below 1%, azotemia can be attributed to decreased IVV, unless the patient has severe liver or cardiac disease causing end-organ hypoperfusion. If severe cardiac failure and severe liver failure (hepatorenal syndrome) can be excluded, the decreased renal perfusion can be assumed to be caused by a decreased effective IVV.
Edema, Ascites, and Pleural Effusion
Effective IVV is increased when edema, pleural effusion, or ascites occurs in the setting of congestive heart failure (CHF). Increased effective IVV cannot be assumed in the presence of edema, ascites, or pleural effusion if there is significant hypoalbuminemia or venous obstruction or if the accumulation of fluid is in a relatively small area of capillary injury (e.g., pleural effusion caused by pulmonary infarction).
Tissue Turgor
Tissue turgor is a function of the elasticity of the solid components of tissue and the degree of distention of the tissues by interstitial fluid. If tissue is depleted of interstitial fluid, it becomes less elastic (i.e., it less readily returns to its original shape after being deformed). Skin turgor is best assessed on the forehead and anterior chest. In patients less than 50 years of age, the turgor of the dorsum of the hand also can be used. In older patients, the elasticity of the solid components of tissue is decreased, and the turgor of the skin becomes unreliable in interpreting changes in interstitial volume.
Central Venous Pressure
The measurement of CVP is a relatively simple but useful means for monitoring cardiac function and cardiovascular status. For the valid measurement of CVP, the catheter must be placed in the large intrathoracic veins near the right atrium (as assessed by chest radiograph), and the catheter must be patent (as assessed by the cyclic variation of CVP with ventilatory movements: decreased CVP during inspiration, increased CVP during expiration).
In normal adults, CVP is about 5 to 12 cm H2O. CVPs below 3 cm H2O are commonly seen in children and young adults who have no evidence of a decreased effective IVV. In older adults and elderly persons, CVP of less than 3 cm H2O can be assumed to reflect a significant decrease in effective IVV.
Central venous pressure is an index of the filling pressure of the right atrium, which, in turn, is an index of the filling pressure of the right ventricle. In uncomplicated circumstances, expansion of the IVV results in increased CVP, whereas contraction of the IVV results in decreased CVP. Central venous pressure cannot be used to assess the adequacy of left ventricular function in patients in whom left ventricular function may be impaired relative to right ventricular function. Central venous pressure also is unreliable when lung disease is present, because it is commonly falsely elevated. In such patients, left ventricular function can be monitored by observing for signs and symptoms of left ventricular failure (dyspnea, development of an audible third heart sound, or pulmonary edema), or by direct measurement of pulmonary capillary wedge pressure. Under normal circumstances, the pulmonary capillary wedge pressure is about equal to the CVP plus 6 mm Hg.
Pulmonary Capillary Wedge Pressure
Technical refinements of the Swan-Ganz catheter make it possible to measure pulmonary artery systolic and diastolic pressure, CVP, pulmonary wedge pressure, and cardiac output using the thermodilution technique with the same catheter. This permits a definitive assessment of the volume status of the patient, because it can be determined whether the cardiac output is appropriate for a given pulmonary wedge pressure. Specific guidelines for the interpretation of the relation between pulmonary wedge pressure and cardiac output are discussed in the following sections.
Patients with Normal Volume Status
Pulmonary wedge pressure can be expected to be between 8 and 12 mm Hg in a patient with a normal cardiopulmonary system and a normal effective IVV. Cardiac output is normal. Pulmonary wedge pressure can be less than 8 mm Hg without indicating volume contraction; in this circumstance, the cardiac output is normal despite the unusually low pulmonary wedge pressure.
Patients Who Are Volume Contracted
Patients who have a normal cardiopulmonary system but who are significantly volume depleted usually have a pulmonary wedge pressure below 8 mm Hg and their cardiac output is less than normal. In patients with chronic pulmonary hypertension (e.g., those with chronic left ventricular failure), a higher than normal pulmonary wedge pressure is needed to drive a satisfactory cardiac output. Thus, in such patients, pulmonary wedge pressure can be above the normal range but be inappropriately low for the patient. This situation can be identified by showing that: (a) cardiac output is less than normal, despite the elevated pulmonary wedge pressure; (b) volume infusion causes an increase in cardiac output toward a more favorable range; and (c) despite further increase in pulmonary wedge pressure with volume expansion, pulmonary function does not deteriorate. (Pao2 does not decrease, PaCO2 does not increase, and pulmonary compliance does not worsen.)
Patients Who Are Volume Expanded
In patients with a normal cardiopulmonary system, pulmonary wedge pressure usually is above 18 mm Hg when volume expansion is substantial. Cardiac output is above normal. If cardiac function is impaired, cardiac output will be inappropriately low for the level of pulmonary wedge pressure.
When a given pulmonary wedge pressure is being interpreted, the serum albumin level also should be taken into consideration, because this opposes the effect of capillary hydrostatic pressure to cause migration of fluid from the capillary lumen to the interstitial space. Thus, at any given elevated pulmonary wedge pressure, pulmonary edema develops more rapidly in a patient who is hypoalbuminemic than in one who has a normal serum albumin concentration. In some patients, it is not possible to obtain a reliable pulmonary wedge pressure. In most of these patients, the pulmonary artery diastolic pressure is a good estimate of the pulmonary wedge pressure. If pulmonary hypertension is present, then pulmonary vascular resistance is increased; thus, pulmonary artery diastolic pressure may not be a good index of the pulmonary wedge pressure. In such patients, it is important to be able to obtain a wedge pressure. Finally, in patients who are being ventilated with high levels of positive end-expiratory pressure, pulmonary wedge pressure may become an unreliable index of left atrial filling pressure because the high intrapulmonary pressures may cause obstruction of the catheter orifice. Patients must be briefly taken off the ventilator for accurate measurements. Other circumstances in which pulmonary artery wedge pressure measurements may be inaccurate include the presence of mitral stenosis or pulmonary venous obstruction.
Blood Pressure
The following guidelines are suggested for the evaluation of the effective IVV from measurement of blood pressure.
1. A nearly normal or expanded effective IVV can be assumed in patients with hypertension that is demonstrated in the sitting or standing position.
2. Effective IVV may be decreased in patients who previously were hypertensive but who have become normotensive.
3. Effective IVV may be decreased in patients who develop orthostatic hypotension (a drop in systolic pressure greater than 10 mm Hg in changing from the supine to the sitting or standing position).
Orthostatic hypotension also can be present, in the absence of volume contraction, as a result of prolonged bed rest, during the use of such antihypertensive agents as methyldopa (Aldomet) or of vasodilators (prazosin, minoxidil). If the pulse rate does not rise as blood pressure falls when a patient stands, autonomic neuropathy should be considered as a cause of postural hypotension.
Systemic Vascular Resistance
Normal values are 50 to 150 dyne-s/cm for pulmonary vascular resistance and 800 to 1,200 dyne-s/cm for systemic vascular resistance. Pulmonary vascular resistance is elevated in hypovolemic shock, cardiogenic shock, pulmonary embolism, or airway obstruction; it is diminished in septic shock. Systemic vascular resistance is elevated in hypovolemic shock, cardiogenic shock, pulmonary embolism, and sometimes in right ventricular infarct and cardiac tamponade; it is decreased in end-stage liver disease and septic shock.