Viral Respiratory Infections Including Influenza
J. Robert Honegger and Michael T. Brady
Infants and preschool children experience 6 to 10 respiratory illnesses per year, and school-age children and adolescents experience 3 to 5 illnesses annually.1 Respiratory infections are predominantly viral infections that are classified into (1) upper respiratory tract infection (URI), meaning the common cold, rhinitis, pharyngitis, otitis media, and conjunctivitis, and (2) lower respiratory tract infection (LRI), namely croup, laryngitis, tracheobronchitis, bronchiolitis, and pneumonia. This section will focus primarily on LRIs occurring in children with viral infections including those associated with characteristic lower respiratory infection presentations, eg, respiratory syncytial virus as the primary cause of bronchiolitis, parainfluenza as the primary cause of croup, and influenza as a primary cause of febrile tracheobronchitis.
Upper respiratory infection is also often associated with viral infection. A large proportion of the otitis media associated with or following a viral respiratory infection is the result of a secondary bacterial infection in the middle ear (see Chapter 243). The likelihood varies dependent upon the particular virus responsible for the associated or antecedent viral respiratory infection. In one prospective study, the risk of developing acute middle ear infection or effusion ranged from 15% to 30% in children with culture-proven viral infections; respiratory syncytial virus infection carried the highest risk.2 However, in many instances, aspirate cultures from middle ears of children with otitis media yield only a respiratory virus. There is also evidence linking bacterial sinusitis to antecedent viral upper respiratory tract infection.
Understanding of the etiologic agents of respiratory infections has changed significantly in the last decade because of widespread application of molecular techniques. Numerous new respiratory viruses have been discovered, including human metapneumovirus, human bocavirus, and several new coronavirus strains. Moreover, the traditional epidemiology of some previously known viruses, such as rhinovirus, has been challenged as a result of polymerase chain reaction assays that exceed the sensitivity of viral culture. Nevertheless, the major associations established by traditional techniques of viral culture, antigen detection, and serology remain valid, eg, respiratory syncytial virus as the primary cause of bronchiolitis, parainfluenza as the primary cause of croup, and influenza as a primary cause of febrile tracheobronchitis.
Generally, specific viral diagnosis from nasopharyngeal aspirates or swabs can be achieved by viral isolation in culture, by rapid viral antigen-detection tests such as immunofluorescence and enzyme-linked immunosor-bent assay, or by nucleic acid amplification tests such as polymerase chain reaction. Retrospective diagnosis may be achieved using serologic methods, but the delay inherent in waiting for a convalescent serum (3–4 weeks) makes this diagnostic method less useful in clinical decision making. The availability of specific antiviral therapies, including neuraminidase inhibitors oseltamivir and zanamivir for both influenza A and B infections, and ribavirin for select cases of respiratory syncytial virus infection, has increased the need for readily available, early, specific diagnosis.
RESPIRATORY SYNCYTIAL VIRUS
Respiratory syncytial virus (RSV) is the foremost cause of viral lower respiratory tract disease in infants and toddlers. Severe disease is generally associated with primary infection, although lower respiratory tract infection can also occur upon reinfection. Bronchiolitis, inflammation of smaller intrapulmonary airways, is the single most distinctive clinical syndrome of lower respiratory tract infection caused by RSV.
 INFECTIOUS AGENT
INFECTIOUS AGENT
Respiratory syncytial virus belongs to the genus Pneumovirus within the Paramyxoviridae family. Other paramyxoviruses include human meta-pneumovirus, mumps, measles, and the parainfluenza viruses. Respiratory syncytial virus is an enveloped, single-stranded, negative-sense RNA virus with a diameter spanning 100 to 350 nm. The viral envelope is studded with spike-like projections that include the fusion (F) and attachment (G) surface glycoproteins, but unlike most paramyxoviruses, the surface proteins of respiratory syncytial virus lack both hemagglutinin and neuraminidase activity. The F glycoprotein mediates fusion of viral particles to target cells and fusion of infected cells to neighboring cells, contributing to syncytium formation. Classification of the two major antigenic subgroups of respiratory syncytial virus (A and B) is based on the heavily glycosylated attachment protein (G). Antibodies specific for both F and G proteins can neutralize viral infectivity. Internal proteins appear to be major targets for cytotoxic T lymphocytes, which are important in terminating established viral infections.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Respiratory syncytial virus has a worldwide distribution. Annual midwinter epidemics of respiratory syncytial virus infection occur in temperate climates; in the United States, epidemics usually begin in November or December and last until March or April. The seasonal epidemicity of infection is less clear in the tropics or subtropical areas (eg, Florida).
Respiratory syncytial virus is the single most common cause of infant hospitalization in industrialized nations, with 70,000 to 130,000 annual infant hospital admissions in the United States. During the first year of life, nearly 15% of infants make outpatient physician visits with respiratory syncytial virus lower respiratory tract infection, and 2% to 3% are hospitalized for respiratory syncytial virus lower respiratory tract infection.3,4 Hospitalization rates have climbed significantly in recent years, possibly in part due to changes in clinical practice and increased day-care attendance. In metropolitan areas, viruses of subgroups A and B frequently cocirculate, although in any given epidemic, one antigenic subgroup may predominate.5 During most epidemics, subgroup A viruses account for the majority of isolates.
Disease in the immediate neonatal period is uncommon; by their first birthday nearly half to two thirds of infants have been infected with respiratory syncytial virus. Seropositivity approaches 100% by age 24 months.6 Reinfection with respiratory syncytial virus occurs throughout life and may occur even during the same winter season. The annual risk of reinfection falls from between 33% and 75% in the preschool years to approximately 20% among school-age children.6 The risk of severe lower respiratory tract infection from reinfection also falls with increasing age and number of exposures, suggesting partially protective immunity.
Risk factors for severe disease include compromised cardiopulmonary function (commonly bronchopulmonary dysplasia or congenital heart disease), premature birth, immunocom-promise, low socioeconomic status, and young age. Boys with severe respiratory syncytial virus lower respiratory tract infection outnumber girls by 1.6 to 1.
The incubation period of respiratory syncytial virus–induced respiratory disease is 2 to 8 days, most commonly 4 to 6 days. Virus can be detected in respiratory secretions up to 4 days before the onset of symptoms and routinely for at least 7 days thereafter. In infants hospitalized with primary respiratory syncytial virus infection, continuous viral shedding for 10 or 11 days is commonly observed; occasional young infants and immunocompromised children have been found to shed the virus for 3 to 4 weeks. Person-to-person spread of respiratory syncytial virus is usually by the droplet route or via fomites; small-particle aerosols do not appear to be very important in the spread of respiratory syncytial virus.7 Without careful attention to infection control procedures, nosocomial infection rates have reached 30% to 40% in children hospitalized 7 days or longer during respiratory syncytial virus epidemics,8 and neonatal unit outbreaks have occurred. Nosocomial transmission is common, so strict hand-washing and use of gown and gloves must be practiced routinely.9
 PATHOGENESIS AND PATHOLOGY
PATHOGENESIS AND PATHOLOGY
Respiratory syncytial virus infection is first established in the upper respiratory tract; initiation of infection is most efficient following inoculation of the nose or eyes.10 Infection may then spread from the oropharynx and nasopharynx to the lower respiratory tract, with clinical evidence of lower tract involvement in approximately 30% of infants experiencing their first infection.6 Respiratory syncytial virus appears to infect predominantly ciliated superficial airway epithelial cells, although nonciliated airway epithelial cells and occasionally alveolar cells may also be involved. In patients who come to autopsy, prominent histopathologic findings include airway plugging with mucus, desquamated epithelium, and inflammatory cells; necrotizing lesions of bronchial and bronchiolar epithelium; and peribronchiolar inflammation with mononuclear cell predominance. The peribronchial infiltrate may extend into the adjacent pulmonary interstitium.
Involvement of the lower respiratory tract decreases with increasing age and repeated infection, so illness is usually restricted to the upper respiratory tract in school-age children. Passively acquired maternal antibody is not adequate to ensure complete protection from respiratory syncytial virus infection and illness. However, children with very high titers of maternally derived neutralizing antibody are at decreased risk for infection and severe illness early in life. These observations have led to use of immunoprophylaxis as a method for protecting children at highest risk of severe respiratory syncytial virus disease. Immunity to respiratory syncytial virus following natural infection with the virus is transient and imperfect, with greater protection against reinfection with homologous than heterologous respiratory syncytial virus subtypes.
With subsequent exposures, reinfection attack rates decline; however, adults are readily reinfected, usually with the production of only upper respiratory symptoms.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
Respiratory syncytial virus infection is heralded by initial symptoms indistinguishable from those of the common cold. The infant may show a nasal discharge and cough, and there may be fever. Within 1 to 2 days, the cough becomes more prominent and tachypnea may develop. With increasing severity of lower respiratory tract infection, the work of breathing increases; substernal and intercostal retractions are noted during inspiration, and active respiratory effort is required during expiration. The expiratory phase is prolonged, and the chest is hyperexpanded and hyperresonant, providing further evidence of generalized expiratory airflow obstruction. Diffuse expiratory wheezing is usually heard, commonly with associated moist crackles.
Apnea can be a relatively early manifestation of respiratory syncytial virus infection in young infants—particularly infants with postnatal age < 8 weeks, history of premature birth, or history of apnea of prematurity. Apnea can occur associated with the respiratory tract symptoms or it may precede the respiratory symptoms.
Radiographs of the lungs reveal hyperexpansion, increased peribronchial markings, and, frequently, areas of atalectasis or infiltrate.
The vast majority of infants have a relatively mild illness and proceed to recover; some, however, may develop significant respiratory compromise. In children requiring hospitalization, hypoxemia is typical, reflecting ventilation-perfusion mismatch. Arterial PCO2 above 45 mm Hg despite tachypnea is indicative of impending respiratory failure and the need to prepare for ventilatory support. Respiratory syncytial virus–related fatality rates for hospitalized children in developed countries are less than 1%. Although children with underlying conditions such as prematurity or cardiopulmonary disease are at increased risk of death, most fatalities occur in previously healthy children. The average duration of hospitalization for previously healthy infants without complications is approximately 2 to 3 days; full recovery may take about 2 weeks.
The association between early childhood severe respiratory syncytial virus lower respiratory tract infection and subsequent recurrent wheezing, reactive airway disease, and pulmonary function abnormalities is under active investigation. Approximately 40% of infants hospitalized with respiratory syncytial virus bronchiolitis later experience repeated wheezing episodes, but many improve by the fifth year of life.11 A tendency toward abnormal pulmonary function tests has been detected through 7 years of follow-up in children previously hospitalized with respiratory syncytial virus as infants.12 It is not known whether respiratory syncytial virus infection plays a causal role in these pulmonary sequelae, or whether it is simply a trigger in predisposed individuals. The agent is nevertheless an important precipitant of wheezing in the child with reactive airway disease.
 DIAGNOSIS
DIAGNOSIS
The American Academy of Pediatrics does not endorse viral diagnostic testing in children with typical bronchiolitis.13 However, obtaining a specific viral diagnosis may be helpful in certain scenarios, such as when patients have risk factors for severe disease, when the diagnosis is uncertain, to reduce unnecessary use of antibiotics, or to control infection. Respiratory syncytial virus (RSV) clinical diagnosis may be achieved from nasal wash or swab specimens by cell culture, antigen detection, or nucleic acid amplification. Cell culture in continuous cell lines such as HEp-2 was traditionally the gold standard for diagnosis, but this technique requires 3 to 7 days for cytopathic effect and is being replaced as the gold standard by the more rapid and sensitive reverse transcriptase polymerase chain reaction nucleic acid amplification tests. Centrifuged shellvial culture with subsequent antigen detection by immunofluorescence offer an alternative to traditional culture, with diagnosis possible in less than 48 hours. Many hospitals rely most heavily on rapid antigen detection systems by immunofluorescence (sensitivity 93–98%, specificity 92–97%) or enzyme immunoassay (EIA; sensitivity 59–97%, specificity 75–100%).14 Serology is not clinically practical for diagnosis of acute RSV infection.
 TREATMENT
TREATMENT
Although most instances of respiratory syncytial virus (RSV) bronchiolitis and pneumonia are mild and self-limited, these illnesses are occasionally severe, and the pediatrician should be aware of three principles in managing the disease. First, RSV bronchiolitis generally peaks in severity within 48 to 72 hours. Therefore, a previously healthy infant seen after this time is at minimal risk of developing more severe disease. Second, the complications associated with hypoxemia and CO2 retention generally begin when the respiratory rate surpasses 60 breaths per minute. Determination of capillary blood O2 saturation by pulse oximetry permits identification of unsuspected hypoxemia. Third, children with community-acquired RSV disease rarely have associated bacterial infections of the lower respiratory tract; antibiotic treatment for lower respiratory tract infection is usually not indicated in these patients. Treatment of bronchiolitis is symptomatic.
Giving humidified oxygen is frequently required when managing hospitalized infants because hypoxemia is common in more severe illness. Cardiorespiratory monitoring, including pulse oximetry, should be considered standard care for young infants with RSV infections. It must be remembered that demonstration of satisfactory arterial oxygen saturation by pulse oximetry does not exclude the possibility of hypoventilation and impending respiratory failure. Apnea is treated with supportive therapy. Mechanical ventilation is often required for approximately 48 hours. Children may manifest periodic breathing for 48 to 72 hours postextubation, but recurrent apnea following RSV-associated apnea is not common, and home apnea monitoring following hospital discharge is usually not indicated. It is particularly important to protect immunocompromised infants, as well as infants with cardiopulmonary disease, from exposure to infants and hospital staff members with RSV infections.
Corticosteroids have not been shown to be effective and are not specifically indicated for RSV infection without evidence of reactive airway disease. In addition, the value of inhaled β-adrenergic agonists such as albuterol or α, β-adrenergic agonists such as racemic epinephrine is not conclusively established and remains controversial. A trial of aerosolized bronchodilator treatment may be employed in managing severely ill children, but there are no data to support the routine use of these agents. A final component of symptomatic care involves assessment for dehydration and provision of oral or intravenous fluids if needed.
The nucleoside analog ribavirin has potent in vitro activity against RSV, but studies of efficacy in humans have yielded mixed results. Some studies have shown modest beneficial effects in parameters such as oxygen saturation, duration of mechanical ventilation, and viral shedding, whereas others have not shown a clear benefit.15 Because of ribivirin’s high cost, difficult delivery requirements, and questionable benefit, current recommendations suggest that it should not be used routinely for RSV bronchiolitis, but may be considered for children with severe RSV disease and those at risk for severe disease due to underlying conditions (immunocompromise, hemodynamically significant cardiopulmonary disease) (see Table 241-1).15
Uncontrolled trials of combination therapy of ribavirin with intravenous immunoglobulin or corticosteroids have been attempted in lung transplant recipients with potential benefit. If ribavirin is to be used, methods to establish rapid RSV diagnosis should be available.
Ribavirin is usually administered for 12 to 20 hours a day for 3 to 7 days. The concentration of ribavirin solution in the nebulizer should be 20 mg/mL of water. Special care must be taken when the drug is administered to children requiring mechanical ventilation, because precipitation of the drug in ventilator tubing or valves can occur, potentially resulting in life-threatening ventilator malfunction. An alternate approach for nonventilated patients uses 2-hour aerosol administration of a ribavirin solution of 60 mg/mL of water given 3 times per day. Without special means to confine or remove ribavirin from the immediate delivery area, room air may be contaminated with ribavirin during administration. Pregnant women should be protected from environmental exposure to the drug because there is teratogenic potential in laboratory animals.
Table 241–1. Recommendations for Chemotherapy of Viral Respiratory Infections
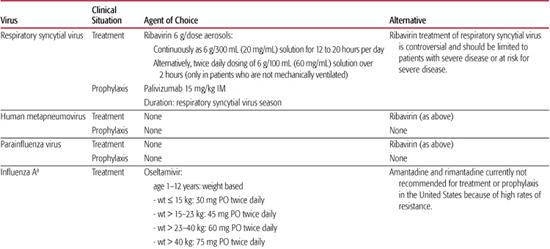
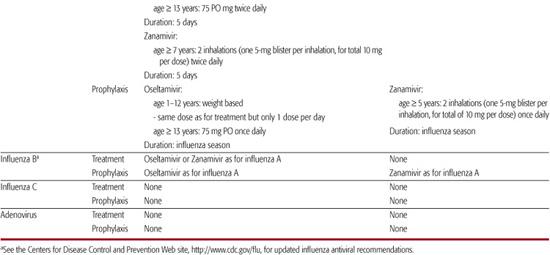
Although antibiotics are not indicated in most children with respiratory syncytial virus (RSV) infections, nosocomial bacterial infections can occur in children receiving intensive supportive care for RSV disease. Additionally, a significant proportion of infants with RSV bronchiolitis have concomitant acute otitis media, which should be managed according to routine guidelines.
 OUTCOMES
OUTCOMES
The association between early childhood severe respiratory syncytial virus lower respiratory tract infection and subsequent recurrent wheezing, reactive airway disease, and pulmonary function abnormalities is under active investigation. Approximately 40% of infants hospitalized with respiratory syncytial virus bronchiolitis later experience repeated wheezing episodes, but many improve by the fifth year of life.11 A tendency toward abnormal pulmonary function tests has been detected through 7 years of follow-up in children previously hospitalized with respiratory syncytial virus as infants.12 It is not known whether respiratory syncytial virus infection plays a causal role in these pulmonary sequelae, or whether it is simply a trigger in predisposed individuals. The agent is nevertheless an important precipitant of wheezing in the child with reactive airway disease.
 PROPHYLAXIS
PROPHYLAXIS
A safe and effective respiratory syncytial virus (RSV) vaccine has not yet been developed. Passive immunoprophylaxis to prevent RSV infection in infants and children at increased risk for severe disease is available with the monoclonal antibody palivizumab. Palivizumab (Synagis) is an intramuscularly administered monoclonal antibody that targets the F glycoprotein of RSV—a surface protein that is highly conserved among RSV isolates. Palivizumab is indicated for the prevention of RSV lower respiratory tract infection in infants and children with chronic lung disease of prematurity, history of prematurity, or congenital heart disease. For specific recommendations, see the current edition of the AAP Report of the Committee on Infectious Diseases (Red Book).15 The 2006 recommendations are that Palivizumab prophylaxis should be considered in infants or children younger than age 2 with chronic lung disease of prematurity who require medical therapy for their chronic lung disease within 6 months of the anticipated start of the RSV season.
Infants who are born prematurely at 32 weeks’ gestation or earlier without chronic lung disease may also benefit from RSV immunoprophylaxis. Infants born at 28 weeks’ gestation or earlier may be candidates for RSV immunoprophylaxis up to age 12 months; infants born between 29 and 32 weeks’ gestation may be candidates up to age 6 months. Considering its high cost, use of palivizumab for infants born between 32 and 35 weeks’ gestation is usually reserved for infants less than age 6 months at onset of the RSV season who have one or more risk factors for severe disease (childcare attendance, school-age siblings, environmental air pollutants, congenital airway abnormalities, or severe neuromuscular disease). Palivizumab is also recommended in children under age 24 months with certain congenital heart conditions (congestive heart failure requiring medication, moderate to severe pulmonary hypertension, and cyanotic heart disease) and certain immunodeficiencies. RSV immunoprophylaxis should be initiated immediately before RSV season, continued monthly during the season, and terminated at the end. Although there is significant regional and year-to-year variability in the onset and duration of the RSV season, in the United States, administration of 5 monthly doses starting in November will usually ensure adequate serum concentrations throughout most of the RSV season.
HUMAN METAPNEUMOVIRUS
First described in 2001, human metapneumovirus (hMPV) is now recognized as a globally distributed respiratory pathogen affecting children and adults. Human MPV may follow respiratory syncytial virus as the second most common cause of lower respiratory tract infections in children.16
 INFECTIOUS AGENT
INFECTIOUS AGENT
Human metapneumovirus (hMPV) was originally isolated from respiratory secretions of 28 children in the Netherlands who had respiratory illnesses ranging from mild rhinorrhea to severe bronchiolitis and pneumonia. The enveloped, 150-to-600-nm virion contains a negative-sense, single-stranded RNA genome and is classified as a member of the Paramyxoviridae family. Like respiratory syncytial virus, which is of the genus Pneumovirus, hMPV has projecting F and G envelope glycoproteins and lacks hemagglutinin activity. However, differences in its genomic sequence and structure result in its placement in the separate genus Metapneumovirus. There appear to be two major groups of hMPV, A and B, which are further divided into four subgroups: A1, A2, B1, and B2.
 EPIDEMIOLOGY
EPIDEMIOLOGY
The hMPV causes respiratory infections across the globe. Though human metapneumovirus was just discovered in 2001, archived serum from the 1950s is seropositive for this agent, suggesting it is not a newly emerging virus. In temperate regions, the virus is present year-round but peaks in late winter and early spring.17 It may account for up to 12% of lower respiratory tract infections and 3% of upper respiratory tract infections in young children, and almost all children are infected by age 5.17 Lower respiratory tract infections from hMPV are usually associated with primary infection and often occur within the first year of life, though generally later than the first respiratory syncytial virus infection. Reinfections occur, though they are generally limited to the upper respiratory tract, suggesting partial immune protection following natural infection. Asymptomatic infections are rare.17 Prematurity, cardiopulmonary disease, and immunosuppression are associated with severe hMPV infection.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
Children infected with human metapneumovirus cannot be distinguished clinically from those infected with respiratory syncytial virus. The most prominent lower respiratory tract infection syndrome of human metapneumovirus is bronchiolitis (59%), followed by croup (18%), exacerbation of asthma (14%), and pneumonia (8%).16 Human metapneumovirus upper respiratory tract infections involve the characteristic symptoms of rhinorrhea, cough, and fever, though fever is less common than with influenza. Acute otitis media may accompany up to 50% of human metapneumovirus infections in young children.
 DIAGNOSIS
DIAGNOSIS
Reverse transcriptase polymerase chain reaction of respiratory specimens is the most sensitive technique for diagnosis of human metapneumovirus. Antigen detection by immunofluorescence using monoclonal antibodies is quite specific, but sensitivity is typically reported as < 75% with these assays.18 Human metapneumovirus growth is fastidious in cell culture, with cytopathic effect often not apparent until after 2 weeks of incubation. Shell vial culture with immunofluorescence staining improves speed and sensitivity over traditional viral culture, but does not reach that of reverse transcriptase polymerase chain reaction.
 TREATMENT AND PREVENTION
TREATMENT AND PREVENTION
Care for most children infected with human metapneumovirus is supportive. Ribavirin is effective against human metapneumovirus in vitro and has been used in severely immunocompromised patients with anecdotal reports of benefit, but no controlled trials have been performed. A number of novel antiviral drugs, monoclonal antibodies, and active vaccine candidates are under investigation for the treatment and prevention of disease by human metapneumovirus.
PARAINFLUENZA VIRUSES
Parainfluenza viruses (PIV) are common causes of acute respiratory infections in young children. Croup is the most distinctive clinical syndrome caused by these agents, although bronchiolitis and pneumonia also occur. The initial infection usually occurs in the first few years of life, and reinfections are common.
 INFECTIOUS AGENT
INFECTIOUS AGENT
The four human parainfluenza viruses have been separated into two genera, Respirovirus (PIV-1 and PIV-3) and Rubulavirus (PIV-2 and PIV-4), within the Paramyxoviridae family. They are large (150–200 nm) enveloped viruses that contain negative-sense, single-stranded RNA. The viral envelope is covered with spikelike projections containing two glycoproteins, the hemagglutinin-neuraminidase (HN) and fusion (F) proteins. These proteins are important for viral attachment and fusion with host cell membranes, and neutralizing antibodies directed against them confer a degree of protection from infection and illness. The four serotypes can be distinguished by type-specific antigens, but the viruses also share common antigens, such that infection with one parainfluenza virus can lead to heterotypic serologic responses to the other serotypes.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Although all four parainfluenza virus (PIV) sero-types are distributed globally and are capable of causing a full spectrum of respiratory illnesses, they tend to occur in distinct epidemiologic and clinical patterns. PIV-1 causes large outbreaks of croup. In the United States, these have occurred for several decades in the fall months of odd years. PIV-2 produces smaller, less severe epidemics of croup in the fall months. PIV-1 and PIV-2 infections only occasionally result in bronchiolitis or pneumonia. PIV-3 regularly causes bronchiolitis and pneumonia, as well as croup. PIV-3 circulates throughout the year with annual outbreaks typically extending from late spring through summer.19 PIV-4 is widely distributed and appears to produce predominantly upper respiratory disease; its epidemiology has not been characterized as thoroughly.
Most children are infected with PIV serotypes 1 to 3 by age 5. Infection with PIV-1 usually occurs between year 1 and 5, whereas PIV-3 occurs earlier, with up to two thirds of infants infected before age 1. Reinfection occurs in children and adults with all serotypes and is usually confined to the upper respiratory tract. Person-to-person transmission of parainfluenza virus occurs via direct contact with large-droplet aerosols or fomites, and illness follows an incubation period of 2 to 6 days.
 PATHOGENESIS
PATHOGENESIS
As with respiratory syncytial virus, initial viral replication is in the upper respiratory tract. Subsequently, there is spread to the lower tract, where viral proliferation within airway epithelium results in cell degeneration and subsequent inflammation. Immunity to infection with parainfluenza viruses is at best incomplete. Infection of adult volunteers revealed that mucosal IgA was better correlated with protection from reinfection than serum IgG. Nevertheless, naturally acquired serum IgG may be protective from lower respiratory tract infection. Progressive disease in certain immunodeficiency states suggests that cellular immunity plays a role in clearing the virus. Studies of hospital epidemics indicate a high attack rate and implicate shedding of virus before the appearance of symptoms. Additional information on pathogenesis of parainfluenza infection can be found in Chapter 224.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
Although most parainfluenza virus infections in children do not progress beyond the upper respiratory tract, in some cases they proceed to cause croup (acute laryngotracheobronchitis), bronchiolitis, or pneumonia. Parainfluenza virus–induced croup begins with an upper respiratory tract infection of several days’ duration, followed by hoarseness and a “barking seal” croupy cough. Inspiratory stridor and marked retractions are evident in more severe infections. Fever is usually mild, only occasionally surpassing 39°C. Most children with viral croup recover after 48 to 72 hours, but some children progress to severe airway obstruction. When croup occurs in children younger than age 6 months or is prolonged or recurrent, underlying anatomic pathology should be suspected. However, recurrent “spasmodic” croup can occur in children with normal airway anatomy. Epiglottitis, usually caused by Haemophilus influenza type b, is distinguished from croup by its abrupt onset of high fever, sore throat, drooling, and stridor that progresses to severe airway obstruction in less than 24 hours. Diphtheria and bacterial tracheitis are also in the differential diagnosis of parainfluenza virus croup.
The bronchiolitis and pneumonia syndromes produced by parainfluenza viruses are clinically indistinguishable from those produced by respiratory syncytial virus. Rare reports implicate parainfluenza virus in cases of meningitis, encephalitis, Guillain-Barré syndrome, and parotiditis.20
 DIAGNOSIS
DIAGNOSIS
Parainfluenza virus (PIV) infection may be suspected in a child with compatible symptoms during a known outbreak, but definitive diagnosis requires identification by culture, antigen detection, nucleic acid amplification, or serology. PIV may be cultured from nasopharyngeal or lower respiratory specimens. These viruses are labile, so care must be taken to promptly place specimens in viral transport media and maintain near 4°C during transport. Antigen detection by immunofluorescent staining or enzyme immunoassay allows rapid diagnosis but has variable sensitivity. Commercially available multiplex polymerase chain reaction assays for detection of type-specific PIV RNA have shown sensitivity and specificity > 95%.21 Retrospective serologic diagnosis can also be achieved, but is limited by cross-reactivity among parainfluenza viruses, as well as with mumps virus.
 TREATMENT AND PREVENTION
TREATMENT AND PREVENTION
Treatment of parainfluenza infections of the respiratory tract is supportive with appropriate management for croup as outlined in Chapter 371. No antiviral medications specifically targeting parainfluenza virus are available. Ribavirin has activity against parainfluenza virus in vitro, and although anecdotal reports have suggested utility in severely immunocompromised children with PIV-3 infection, cohort studies have not found a mortality benefit.22 No parainfluenza virus vaccine is available, but numerous live attenuated and subunit vaccines are under investigation.
INFLUENZA VIRUSES
Influenza is an acute respiratory illness typically accompanied by fever and systemic symptoms. Annual global epidemics interspersed with occasional pandemics result in considerable morbidity and mortality in children and adults. The recent development of an avian influenza strain capable of causing highly lethal disease in humans has heightened public awareness of influenza and its potential as a global health threat.
 INFECTIOUS AGENT
INFECTIOUS AGENT
The influenza viruses are members of the Orthomyxoviridae family and separated into the three genera Influenzavirus A, Influenzavirus B, and Influenzavirus C. Type A influenza viruses are maintained in birds and circulate among mammals such as humans, swine, and horses. The enveloped virion is about 100 nm in diameter and studded with spikelike projections consisting of its surface glycoproteins hemagglutinin (HA) and neuraminidase (NA). The inner surface of the envelope is lined by the matrix protein (M1). The core ribonucleoprotein (RNP) complex encompasses a negative-sense, single-strand, segmented RNA genome. Each of the eight RNA segments codes for 1 or 2 of 11 viral proteins. The segmented nature of the influenza genome allows for exchange of RNA segments when two different influenza virions infect the same cell (genetic reassortment). This property has great significance for the epidemiology of pandemic influenza.
Influenza A viruses are divided into subtypes based upon their hemagglutinin (HA) and neuraminidase (NA) surface antigens. Major influenza A subtypes circulating in humans have been A(H1N1) and A(H3N2), and most recently the reassortment virus A(H1N2). Protective antibodies directed against particular subtypes of HA or NA are of little or no cross-protective benefit against alternative subtypes. Up to 16 HA subtypes and 9 NA subtypes have been identified in birds. Influenza B and C have less antigenic variability and are not divided into subtypes.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Since influenza A(H1N1) reemerged in 1977, each annual winter influenza epidemic has been dominated by one or two of the major globally circulating viruses: A(H1N1), A(H3N2), and type B. A(H3N2) epidemics have tended to be the most severe. Although a degree of subtype-specific immunity develops after infection, accumulating mutations in the hemagglutinin and neuraminidase genes (“antigenic drift”) produce new variants to which the individual may again be susceptible, perpetuating the annual epidemics.
At varying intervals, major changes in the hemagglutinin and neuraminidase surface antigens of influenza A strains (“antigenic shift”) occur because of genetic reassortment of RNA segments between human and animal viruses or adaptation of a new animal viruses to humans. These events, which occurred three times during the 20th century, render antibody to previously circulating influenza A viruses unprotective. Worldwide pandemics associated with considerable excess mortality then ensue. The highly pathogenic avian influenza A(H5N1) has caused 348 confirmed human infections with a mortality rate of 62% between 2003 and 2007,23 sparking widespread concern that a devastating pandemic could erupt if this or a similarly pathogenic strain were to gain the capacity for efficient human-to-human transmission. In March 2009, Mexico experienced outbreaks of respiratory illness, including influenza-like illness, that was subsequently identified to be caused by novel swine-origin influenza A(H1N1) virus (S-OIV).24 From March 1, 2009 through April 30, 2009, Mexico reported a total of 1918 suspected cases with a total of 84 deaths (mortality rate of 4.4%).24 At approximately the same time, influenza-like illness was being reported in the United States and subsequently countries on all continents.25 The rapid transmission around the globe and relatively high mortality rate in Mexico created concern that the S-OIV H1N1 represented a new pandemic strain with marked global implications. As of May 29, 2009, the United States had 8,975 confirmed and probably cases with 15 deaths (mortality rate of 0.16%).26 Many of those who died had medical conditions that had been previously identified as being associated with an increased risk for influenza complications. The illness and mortality rates noted in the United States and other countries were more consistent with seasonal influenza. However, since this is a novel strain, close monitoring and global cooperation to reduce transmission is prudent. This S-OIV H1N1 strain is a recent reassortant of two strains: a triple-reassortant swine influenza A(H1) virus that has been circulating in pigs for more than a decade and a Eurasian swine influenza virus.27 The triple-reassortant swine influenza A(H1) has been occasionally transmitted to humans, but has not spread efficiently from human to human. S-OIV H1N1 strains are not epidemic in pigs at this time but clearly are capable of human to human transmission.27
In the United States, interpandemic influenza activity usually peaks between December and March, though occasionally as early as November or as late as May. The annual epidemics result in an estimated 226,000 hospitalizations and 34,000 deaths in the United States—the majority of deaths occurring in the elderly.28 During influenza season, the virus accounts for 10% to 19% of outpatient visits for children under age 5 with acute respiratory infections or fever.29 Hospitalization rates are highest for children under age 6 months. Young children excrete more virus for a longer duration than their older counterparts. The virus is highly contagious, transmitted primarily by aerosolized droplets formed by coughing or sneezing, though contact with contaminated surfaces can also spread the virus. Symptoms follow after a 1- to 4-day incubation period.
 PATHOGENESIS
PATHOGENESIS
Influenza infection is initiated by virus inoculation in the upper or lower airways. If present, virus-specific IgG and IgA antibodies against the surface antigens, particularly hemagglutinin, may interrupt infection or illness. The viral hemagglutinin molecule allows viral attachment and later membrane fusion with respiratory epithelial cells. Neuraminidase facilitates penetration through the sialyloligosaccharide-rich respiratory mucous layer for access to the epithelial cells and later is important for release of newly packaged viruses from cells. Upon infection and replication in the respiratory epithelium, virus is shed into the respiratory secretions and local spread ensues, with eventual desquamation. The entire airway from pharynx to alveoli may be involved. Diffuse pneumonia due to alveolar infection can be life-threatening.
Viral infection remains, for practical purposes, limited to the respiratory tract. With the exception of A(H5N1) disease, viremia is extremely rare. Systemic symptoms are believed to be manifestations of inflammatory mediators. Influenza infection results in damaged mucociliary function, reduced neutro-phil function, and other immune impairments that are probably responsible for the increased risk of bacterial superinfection observed with influenza infections.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
Influenza in children typically presents with sudden fever and respiratory symptoms. Fever is more prominent than with other viral respiratory infections, occurring in 95% of children, routinely reaching 39°C (102.2°F) to 40°C (104°F), and lasting 3 to 6 days in the absence of complications. In older children, the typical “flulike syndrome” consists of initial fever, headache, myalgias, malaise, sore throat, and dry cough, followed later by a more productive cough. A tracheobronchitis develops, often with a normal chest exam and radiograph. Children under age 5 usually present with a febrile upper respiratory tract infection, but some manifest severe croup, bronchiolitis, or pneumonia.29 The novel swine-origin influenza A(H1N1) strain S-OIV that was first detected in the United States in the spring of 2009 has been associated with a clinical illness that is similar to other seasonal influenza strains. Neonatal influenza may mimic bacterial sepsis. Acute otitis media affects approximately 1 in 4 children with influenza29
Three patterns of pneumonia are seen with influenza A or B virus infections: viral bronchopneumonia, diffuse viral hemorrhagic alveolitis, and secondary bacterial pneumonia. Viral bronchopneumonia is typically mild, rarely leading to severe disease and death in otherwise healthy children. Pandemic strains and the recent avian A(H5N1) strains have been particularly capable of causing a dangerous hemorrhagic alveolitis—manifesting on days 3 to 5 of illness with severe dyspnea, cyanosis, blood-tinged sputum, and radiographic findings suggestive of pulmonary edema. Superinfecting bacterial pneumonia usually occurs after 5 to 7 days of illness with recurrent fever and pulmonary symptoms; causative agents include Streptococcus pneumoniae and Staphylococcus aureus most commonly, followed by Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pyogenes. S aureus bacterial tracheitis and toxic shock syndrome may also complicate influenza infection.
Other complications of influenza infection include Reye syndrome (usually in association with aspirin use), myositis, myocarditis, encephalopathy, and postinfectious encephalitis.
 DIAGNOSIS
DIAGNOSIS
Influenza may be cultured from respiratory secretions obtained by throat swab or nasal lavage upon inoculation into monkey or canine kidney cell cultures or embryonated eggs. Virus growth can be detected after 2 to 6 days by hemadsorption, hemagglutination, or on occasion by evidence of cell destruction. Specimens for viral isolation will have a higher yield when obtained in the first 72 hours of clinical illness. Antigen detection by immunofluorescence or enzyme immunoassay and RNA detection by reverse transcriptase polymerase chain reaction permits diagnosis more rapidly. A large number of commercial rapid detection assays have been developed that provide results within 30 minutes for use in clinical decision making. Although these assays are relatively specific (median 90–95%), sensitivities are low (median 70–75%) and therefore their use is recommended only during periods when influenza is prevalent.26
 TREATMENT
TREATMENT
In general, influenza infection in children is a self-limited condition. Supportive care includes antipyretics other than aspirin to reduce fever and discomfort. Salicylates should not be used to treat influenza-related symptoms in children or adolescents because of the increased risk of Reye syndrome. Bed rest and maintenance of adequate fluid intake may also provide comfort.
Antiviral therapies are discussed in Chapter 245, and use in specific respiratory infections is outlined in Table 241-1. The Centers for Disease Control and Prevention (CDC) updates recommendations for influenza antiviral use annually.30 Zanamivir and oseltamivir are neuraminidase inhibitors that are effective for both the treatment and prevention of influenza A and influenza B illness. Zanamivir is approved for treatment in children over age 7 and is given at the same dose as for adults: two inhalations twice daily (one 5-mg blister per inhalation, for a total of 10 mg per dose). Oseltamivir is approved for treatment in children over age 1. Dosage is detailed in Table 241-1. Treatment in uncomplicated influenza reduces symptom duration only if initiated within 48 hours of onset of illness, and should be given for a 5-day course. Resistance to neuraminidase inhibitors can occur but is uncommon at the present time. Zanamivir is not recommended for use in children with underlying airway disease because of associations with bronchospasm. Oseltamivir occasionally causes nausea. Neuropsychiatric events due to oseltamivir have been reported.
Because of markedly increasing viral resistance to amantadine and rimantadine, use of these agents is not currently advised. By 2008, increasing resistance to oseltamivir in a certain influenza A H1N1 strain was noted (99.2% resistance of A/Brishane/59/2007 that was circulating in 2008–2009 season)31 This strain was still sensitive to zanamivir, also a neuraminidase inhibitor, and sensitive to amantadine and rimantadine. However, the novel swine-origin influenza A H1N1 strain (S-OIV) identified in Spring 2009 was sensitive to both oseltamivir and zanamivir; but was resistant to amantadine and rimantadine. At all times, it would be prudent to refer to the CDC Web site to monitor the most appropriate antiviral regimen for circulating influenza strains. At the present time, zanamivir alone or a combination of oseltamivir plus rimantadine would be appropriate as empiric treatment if susceptibilities of antiviral agents to circulating strains were not available.30,31 Both influenza A and B viruses are sensitive in vitro to ribavirin, and some studies suggest clinical benefit with aerosolized administration. However, this approach is not approved or recommended by the Food and Drug Administration.
 PROPHYLAXIS
PROPHYLAXIS
Immunoprophylaxis with trivalent inactivated influenza vaccines (TIVs) and live attenuated, cold-adapted influenza vaccines (LAIVs) and chemoprophylaxis using neuraminidase inhibitors are available measures that are effective in reducing the number of influenza virus infections and in reducing the impact of influenza disease.
Each year, trivalent inactivated influenza vaccines and live attenuated, cold-adapted influenza vaccines are modified to contain antigens from three strains that are predicted to circulate during the following influenza season: one influenza A(H1N1) virus, one influenza A(H3N2) virus, and one influenza type B virus. The viruses for both vaccine forms are propagated in eggs, and both are contraindicated in individuals with a history of severe anaphylactic reaction to eggs. Trivalent inactivated influenza vaccine is approved for use in individuals > 6 months old. Live attenuated, cold-adapted influenza vaccine is approved for use in individuals ages 2 to 4 who lack a history of recurrent wheezing and for healthy, nonpregnant individuals ages 5 to 49. For children with little prior experience with influenza (< age 9), two separated doses of the influenza vaccine should be administered for their initial immunization in order to ensure a satisfactory antibody response. Currently, immunization strategies focus on individuals who are most likely to experience complications following influenza and those who are likely to be a source of spread of the virus to at-risk populations (eg, health care workers and household contacts). See http://www.cdc.gov/flu for the Public Health Service Advisory Committee on Immunization Practices (ACIP) updated recommendations on influenza vaccine target populations, dosage, administration instructions, and adverse effects.
The duration of protection from the inactivated influenza vaccine is brief, likely less than 1 year. Patients who have chronic diseases or immune deficiencies may develop lower postvaccination antibody titers. Some of these individuals may remain susceptible to influenza-related respiratory infections despite receiving an appropriate influenza vaccine. These patients are still candidates for influenza vaccination because their immune response to the vaccine may be adequate to prevent a more serious complication associated with lower respiratory tract involvement. For further discussion of influenza vaccination see Chapter 244.
Most experience with chemoprophylaxis has been with rimantadine and amantadine. However, given development of significant resistance, the neuraminidase inhibitor oseltamivir is now the primary agent recommended for children. It is approved for use in children over age 1. Zanamivir may also be used and is approved for children over age 5 as a prophylactic agent.
Chemoprophylaxis may be beneficial for specific groups of children, but should not be considered a substitute for influenza vaccination. Candidates for chemoprophylaxis include individuals at high risk of complication who were not fully vaccinated (2 doses received for children < age 9 receiving vaccine for the first time) more than 2 weeks before exposure, individuals with immunodeficiency who might not be able to develop protective antibody responses after vaccination, and unvaccinated contacts of high-risk individuals. Chemoprophylaxis is also effective during institutional influenza outbreaks. For children for whom chemoprophylaxis is deemed appropriate, it should be instituted at the onset of the influenza virus outbreak and continued throughout the entire influenza season. In most locations, this is approximately a 6-week period.
Nosocomial influenza infection may be a serious concern within facilities caring for children. Transmission of influenza to children at risk for influenza-related complication can occur from contact with infected patients, infected health care workers, and infected family members. Staff members who develop influenza illness should be required to stay home from work. Visitors, including family members with any illness consistent with influenza virus infection, should avoid or minimize contacts with patients at high risk for influenza complications. A hospital-wide visitation restriction may be necessary in certain circumstances if influenza virus activity in the community is extremely high. Any visitor who has a febrile respiratory illness should be restricted from visiting. Children identified as having an illness consistent with influenza, regardless of laboratory test results, should be isolated in single rooms or cohorted. Precautions should include droplet isolation. Hand-washing is essential. Respiratory secretions should be considered infectious material. Gowns and gloves should be used when touching infected material or if soiling is likely. Because small-droplet aerosols are responsible for transmission, masks or face shields should be used when within 3 feet of a patient to reduce the likelihood of acquisition of influenza infection by health care workers or by visitors. Special air handling is not required because the droplets do not remain suspended in the air. During an influenza outbreak, it may be prudent to postpone elective surgery, particularly for patients who have any symptoms of an influenzalike illness (community acquired or nosocomially acquired).
ADENOVIRUSES
Adenoviruses account for 5% to 10% of respiratory illnesses in children and are important causes of ocular, gastrointestinal, and urologic infections. They are also recognized as serious pathogens in immunocompromised hosts.
 INFECTIOUS AGENT
INFECTIOUS AGENT
The adenoviruses are members of the Adenoviridae family in the genus Mastadenovirus and are divided into six species (A–F) on the basis of their DNA sequence. There are now 51 recognized human adenovirus serotypes. They are nonenveloped DNA viruses that are icosahedral in shape and measure 70 to 80 nm in diameter. Antigenically important coat proteins include hexon, penton base, and fiber.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Care must be taken in associating the isolation of adenoviruses with disease entities, because these viruses are capable of establishing persistent or latent infection in the tonsils, adenoids, and intestines of infected children, and recurrent excretion can occur. Most children are infected with sero-types 1, 2, and 5 during the early years of childhood. Serotypes 3, 6, 7, and 21 are less frequent but important causes of respiratory infection during childhood. Other serotypes with distinctive disease associations are described below.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
Respiratory Illness
Infants and children most commonly manifest upper respiratory disease symptoms: coryza, conjunctivitis, otitis media, and pharyngitis. Exudative tonsillitis may be present, and systemic symptoms such as fever, malaise, and headache may be prominent. Adenovirus 1, 2, 3, and 5 are common contributors.32 The respiratory symptoms may be accompanied by conjunctivitis, in a condition termed pharyngoconjunctival fever, and commonly caused by adenovirus 3. Bronchiolitis or pneumonia occurs in a small percentage of children with infections. Although most children recover without complications, some outbreaks caused by serotypes 3, 7, and 21 have resulted in fulminate disease with significant mortality. Disseminated disease with exanthem, meningoencephalitis, hepatitis, and rhabdomyolysis has been reported. Finally, adenoviruses have been associated with a pertussislike syndrome.
Epidemic Keratoconjunctivitis
In this more serious form of ocular adenovirus infection, conjunctivitis is followed by corneal infiltrates and enlargement of preauricular nodes. Adenovirus serotypes 8, 19, and 37 are major causes.
Extrarespiratory Manifestations
The enteric adenoviruses, serotypes 40 and 41, are important causes of infant diarrhea. Adeno-viruses (not necessarily the enteric serotypes) have been recovered from mesenteric lymph nodes of children with intussusception, suggesting a role for these viruses in the pathogenesis of some intussusception. Adenovirus types 11 and 21 are associated with acute hemorrhagic cystitis, producing hematuria from several days’ to 2 weeks’ duration. Viral myocarditis is occasionally associated with adenovirus. Neurologic disease, principally meningoencephalitis, is attributed to adenovirus infection. Similarly, adenovirus infection may cause severe hepatitis and liver failure, particularly in infants or immunocompromised patients. Finally, severe disseminated adenovirus diseases with detectable viremia has been seen in immunocompromised patients, particularly hemata-poetic stem cell transplant recipients.
 DIAGNOSIS
DIAGNOSIS
Adenoviruses can be identified in nasopharyngeal secretions, oropharynx swabs, eye swabs, urine, and feces by traditional culture in primary or permanent cell lines, shell vial culture, antigen detection, and polymerase chain reaction. Because virus excretion from the pharynx may persist for weeks or even chronically following adenovirus infection, the diagnostic significance of adenovirus isolation (particularly the common childhood types 1, 2, and 5) from respiratory and fecal samples is not as strong as it is with many of the other respiratory viruses. In these cases, serologic evidence of increasing antibody titers may be useful to confirm recent infection. Antigen detection is generally less sensitive than culture but provides a rapid diagnosis.
In post-hematopoietic stem cell transplant patients, monitoring adenovirus viremia by polymerase chain reaction may be useful for detecting patients at risk of dissemination.
 TREATMENT AND PREVENTION
TREATMENT AND PREVENTION
For most children, treatment is symptomatic. The nephrotoxic nucleotide analog cidofovir has shown mixed results in immunocompromised hosts, based upon uncontrolled reports. More effective prodrugs are in development. An effective, live, enteric-coated oral vaccine against serotypes 4 and 7 has been used in military recruits, but has not been employed in civilian populations.
RHINOVIRUSES
Long known as the major cause of “common colds” in adults and children, rhinoviruses are also recognized as important triggers of recurrent wheezing. Accumulating evidence suggests that rhinoviruses may have a role in pediatric lower respiratory tract infections as well.
 INFECTIOUS AGENT
INFECTIOUS AGENT
Rhinoviruses are small (20–30 nm), non-enveloped, icosahedral, RNA viruses belonging to the Picornaviridae family. Most replicate optimally at 34°C (93.2°F) to 35°C (95°F), the temperature of the mucosa of the upper respiratory tract. There are roughly 100 different human rhinovirus serotypes, with little cross-protection between types.
 EPIDEMIOLOGY AND CLINICAL MANIFESTATIONS
EPIDEMIOLOGY AND CLINICAL MANIFESTATIONS
Rhinovirus is most associated with the common cold—the illness dominated by nasal obstruction or discharge, with accompanying cough, sore throat, and mild or absent fever. Rhinovirus has also been established as a trigger of wheezing in children with asthma. Despite its temperature-sensitive replication, a number of lines of evidence now suggest that rhinovirus may be able to replicate in the lower airways and may be a significant cause of bronchiolitis and pneumonia in children.33 Causality of lower respiratory tract infection by rhinovirus remains controversial, however, in part because rhinovirus is relatively frequently detected in asymptomatic individuals. Rhinovirus upper respiratory tract infection is associated with the development of otitis media. Infection frequency is greatest during the fall months. Transmission occurs primarily by the hand-to-nose route and by large-droplet spread. Hand-washing and avoidance of hand contact with respiratory secretions of affected individuals reduces viral transmission.
 DIAGNOSIS AND TREATMENT
DIAGNOSIS AND TREATMENT
Specific viral diagnosis is rarely necessary for the clinical management of rhinovirus infections. When available, reverse transcriptase polymerase chain reaction is significantly more sensitive than cell culture for the diagnosis of rhinovirus. No specific antiviral treatment is currently available for rhinovirus infections. Over-the-counter cough and cold preparations do not provide significant benefit for children with the common cold and are not recommended. Vitamins (ascorbic acid), minerals (zinc), and herbal remedies (Echinacea) are frequently used for the treatment and prevention of the common cold, with limited evidence of any benefit in reducing symptoms.
CORONAVIRUSES
Coronaviruses have long been identified as causes of common colds in adults and children. Emergence of the highly lethal severe acute respiratory syndrome (SARS) coronavirus in 2002 brought heightened attention to this group of viruses. Technologies developed during the SARS epidemic contributed to the recent discovery of two additional strains of coronavirus such that four non-SARS coronaviruses are currently known to circulate among humans. The coronaviruses are enveloped, pleomorphic, RNA-containing viruses that measure about 120 nm in diameter.
Coronaviruses may be responsible for about 5% to 18% of childhood respiratory infections.34 Most of these infections are upper respiratory tract infections, although some cases of croup, bronchiolitis, and pneumonia have been described.35 Non-SARS coronavirus epidemics have occurred. The 2002–2003 SARS epidemic affected 8000 people in 29 countries with a case fatality rate approaching 10%.35 The growth of coronaviruses in tissue culture is limited, and reverse transcriptase polymerase chain reaction has enhanced the ability to detect the viruses in infected individuals. Treatment is symptomatic, as it is for rhinovirus infections.
BOCAVIRUS
Human bocavirus, of the family Parvoviridae, was discovered in 2005 using novel molecular techniques in children with respiratory infections. Subsequent analyses have found this virus in approximately 5% of young children with acute respiratory infections, with manifestations including wheezing and pneumonia.29 The virus is most frequently identified in children < age 2. The virus has also been identified in the stool of some children with gastroenteritis. Although this agent is not commonly found in asymptomatic individuals, its causal role in childhood acute respiratory infections has been called into question because of the high frequency of coinfection with other viruses and the potential that the virus may persist asymptomatically in some individuals. Serologic techniques are not currently available to correlate antibody responses to symptoms, and no culture techniques or animal models are available for testing of Koch’s postulate. Nevertheless, the recent finding that bocavirus viremia is detected in individuals with more severe respiratory symptoms argues that the virus may be a true pathogen causing lower respiratory tract infection in children. Reverse transcriptase polymerase chain reaction is the only currently available diagnostic modality for bocavirus infection, and treatment is supportive.
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


