Venous Thromboembolism in Pregnancy
Patricia M. Witcher
Lewis Hamner
Venous thromboembolism (VTE) is a disease process that collectively refers to deep vein thrombosis (DVT) and pulmonary embolism (PE). While the incidence of VTE during pregnancy is unknown, there is a general belief that pregnancy predisposes women to VTE because of changes in the coagulation system during pregnancy. Events and procedures commonly performed during pregnancy have a propensity for thrombogenesis. An understanding of the significance of VTE, physiology of coagulation, and predisposing risk factors facilitates the medical provider and the professional nurse in the identification of women at risk so that appropriate evaluation and treatment can be employed.
Incidence and Significance
As many as 30 to 50 percent of DVTs are unrecognized and, if untreated, DVT may progress to PE in about 15 to 25 percent of patients.1,2,3 DVT occurs with the same or higher frequency during the antepartum period as the postpartum period.4,5,6 The risk for PE is greatest during the postpartum period.3,6,7 PE is of ultimate concern because of the mortality risk. It remains one of the leading causes of death in pregnant women.8 Morbidities associated with VTE include pulmonary hypertension (following recovery from an acute PE event), recurrence of VTE, and increased risk for venous insufficiency from compromised blood flow to the affected limb.9,10
The incidence of VTE in pregnant women is estimated to be 0.5 to 3 per 1,000 pregnancies across all reproductive ages.4,11,12,13,14,15 This incidence is somewhat higher than what is reported for the general population, which is estimated to be about 1 to 1.45 per 1,000 adults each year.16,17 The incidence of VTE in the general population varies based upon ethnicity. Individuals of Asian Pacific or Hispanic descent have a 2.3- to 4-fold lower risk than Caucasians and African Americans.16 The true incidence of VTE in pregnant women is unknown, but there is a general belief that pregnancy is associated with as much as a two- to sixfold increase in VTE risk. 3,4,18,19 Vascular and hemostatic changes of pregnancy may account for a potentially increased risk.
Physiology of Coagulation
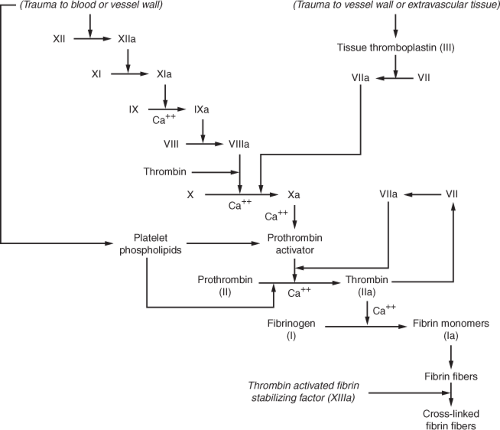
Normal coagulation requires a balance between procoagulants and anticoagulants. Anticoagulants usually exceed procoagulants in the circulation until a triggering mechanism, such as vascular or tissue injury, activates coagulation.20 The complex system of coagulation relies upon further interaction of anticoagulants and procoagulants so that excessive coagulation does not occur. Typically, injury to the vessel wall or to the red blood cells initially results in vasoconstriction to minimize the surface area that requires clotting and platelet activation. Following platelet activation, platelets adhere to the damaged vessel wall and secrete multiple substances that perpetuate further platelet adherence, platelet aggregation, and activation of clotting factors.21 What ensues is a complicated sequence of activation of clotting factors with the end result being formation of a stable clot. Figure 17-1 illustrates normal coagulation.
Vascular or tissue trauma, or trauma to the blood itself, may initiate the clotting cascade by what has been referred to as the intrinsic and extrinsic pathways. The intrinsic pathway begins with activation of factor XII. Activated factor XII (XIIa) then activates factor XI (XIa), which cleaves factor IX to form activated factor IX (IXa), which, in turn, activates factor VIII (VIIIa). Factor X becomes activated (Xa) by factor VIIIa as well as from activated factor VII (VIIa) along with the cofactor, tissue thromboplastin, which propagates coagulation via the extrinsic pathway. The extrinsic pathway is also stimulated by vascular or extravascular tissue injury and begins with tissue thromboplastin (factor III). Both the intrinsic and extrinsic pathways converge to the
common pathway, which begins with the activation of factor X (Xa). Prothrombin activator enzymatically cleaves prothrombin into thrombin. Thrombin facilitates further activation of factors V, VIII and XIII and cleaves fibrinogen into fibrin, which combines with other fibrin monomers to form fibrin fibers. Thrombin activates fibrin stabilizing factor (XIIIa), which is released from platelets that are entrapped in the fibrin clot, in order to promote a stable clot by promotion of cross-linkages with and between other fibrin fibers.20
common pathway, which begins with the activation of factor X (Xa). Prothrombin activator enzymatically cleaves prothrombin into thrombin. Thrombin facilitates further activation of factors V, VIII and XIII and cleaves fibrinogen into fibrin, which combines with other fibrin monomers to form fibrin fibers. Thrombin activates fibrin stabilizing factor (XIIIa), which is released from platelets that are entrapped in the fibrin clot, in order to promote a stable clot by promotion of cross-linkages with and between other fibrin fibers.20
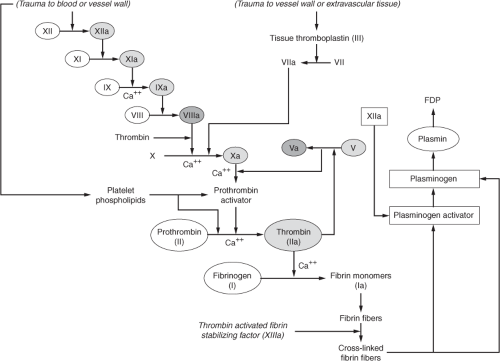
Several clotting inhibitors are stimulated by certain clotting factors in an effort to regulate thrombus formation. Figure 17-2 depicts the process responsible for regulation of coagulation. A deficiency or impaired activity in these inhibitors results in excessive clotting. Thrombin that does not absorb to fibrin fibers combines with antithrombin (AT III). In addition to inactivating thrombin, AT III further blocks the activation of fibrinogen. When heparin, which normally exists in low concentrations in the blood, increases in the circulation (i.e., pharmacologic administration) it combines with AT III and removes thrombin as well as activated factors XII, XI, IX and X.20 Plasminogen activator gradually becomes activated by factor XIIa and converts plasminogen—which is trapped inside the clot—into plasmin, which in turn promotes lysis of the clot. The digestion of fibrin by plasmin yields fibrin degradation products (FDPs), also known as fibrin split products (FSPs), measurement of which provides some evidence of fibrinolysis.21 Plasmin assists in the regulation of coagulation by digesting coagulation factors XII, VIII, V, and prothrombin.20 Thrombomodulin, a protein normally bound to the endothelial membrane that specifically binds to thrombin, is another mechanism that prevents further extension of the clot beyond what is required in order to promote hemostasis at the site of vascular injury. The thrombomodulin–thrombin complex, along with protein S, activates protein C, resulting in activated protein C (APC). APC, along with cofactor
protein S, inactivates factors VIII and V to promote anticoagulation and perpetuate the balance between procoagulants and anticoagulants.
protein S, inactivates factors VIII and V to promote anticoagulation and perpetuate the balance between procoagulants and anticoagulants.
Hemostatic Changes During Pregnancy
Rudolf Virchow, a German pathologist, identified three primary mechanisms (Virchow’s triad) that lead to thrombosis. These are illustrated in Figure 17-3 and include endothelial damage, venous stasis, and hypercoagulability.
Some degree of vascular damage may occur during vaginal delivery or Cesarean section. Endothelial damage promotes exposure of blood to tissue thromboplastin and activation of the components of the coagulation cascade, although this plays less of a role than the hormonal and hemostatic changes of pregnancy in promoting thrombosis.16,22 Hormonal and hemodynamic changes of pregnancy contribute to venous stasis because of the increase in circulating blood volume combined with hormonally mediated venous distention and increased blood capacity in the periphery.3,4,9,22 Venous return is further obstructed by the gravid uterus.2,3 Reduction in the velocity of blood flow occurs in the lower extremities, starting early in the first trimester and continuing throughout pregnancy and the postpartum period. The decreased blood velocity is more pronounced in the left leg than the right and is thought to result from increased
compression of the left common iliac vein by the right iliac artery, which crosses on the left side only.3,4,9 Venous stasis is believed to predispose to thrombus formation because blood stasis reduces clearance of activated clotting factors, thus allowing them to deposit in muscular venous network or in the valve cusp pockets of the deep veins.6,23 The interaction between the procoagulants and anticoagulants further determines if any thrombus that has formed extends.
compression of the left common iliac vein by the right iliac artery, which crosses on the left side only.3,4,9 Venous stasis is believed to predispose to thrombus formation because blood stasis reduces clearance of activated clotting factors, thus allowing them to deposit in muscular venous network or in the valve cusp pockets of the deep veins.6,23 The interaction between the procoagulants and anticoagulants further determines if any thrombus that has formed extends.
Pregnancy-related changes in the coagulation system promote a hypercoagulable state with increases in clotting factors such as factors VIII, VII, X and fibrinogen and decreases in inhibitors of clotting.3,4,5,11,22,24,25 These changes are summarized in Figure 17-4. Thrombin generation therefore increases. There are decreases in protein S and increased resistance to activated protein C which contribute to hypercoagulability.3,5,9,14,22,24,25 Fibrinolysis is
also reduced, perhaps secondary to a reduction in the activation of plasminogen activator which occurs as a result of decreases in plasminogen activator inhibitors 1 and 2 (PAI-1 and PAI-2).3,4,5,14,22,25 The latter is derived from the placenta.4
also reduced, perhaps secondary to a reduction in the activation of plasminogen activator which occurs as a result of decreases in plasminogen activator inhibitors 1 and 2 (PAI-1 and PAI-2).3,4,5,14,22,25 The latter is derived from the placenta.4
Table 17.1 Inherited Thrombophilias in Pregnancy | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Risk Factors for VTE
VTE during pregnancy is multifactorial and arises from a complex interaction between the hemostatic changes of pregnancy, underlying thrombophilia, and additional acquired risk factors. In general, women at highest risk for VTE are those with an inherited thrombophilic condition and a prior history of VTE. Thrombophilia may be inherited or acquired; the prevalence of the different thrombophilias varies with regard to ethnicity and thrombogenic potential as well as the predisposition to adverse pregnancy events.26 The most common acquired thrombophilia is antiphospholipid antibody syndrome (APS), which is characterized by the presence of antiphospholipid antibodies, lupus anticoagulant (LAC), and anticardiolipin antibody (ACLA) with arterial and/or venous thrombosis. There are multiple inherited thrombophilias with varying propensities for thrombosis based upon the specific abnormality. These are listed in Table 17-1. In general, the thrombophilias associated with the highest incidence of VTE are the least common during pregnancy. The thrombophilias that are considered to be the most thrombogenic without a personal or family history of VTE are compound heterozygous factor V Leiden and prothrombin G20210A mutation (inheritance of both thrombophilias), homozygous factor V Leiden mutation, the homozygous form of prothrombin G20210A mutation, and AT III deficiency.9,26,27
Table 17.2 Risk Factors for Venous Thromboembolism During Pregnancy | ||||||
|---|---|---|---|---|---|---|
| ||||||
Women with a previous VTE are considered to be at increased risk for recurrence of VTE during a subsequent pregnancy; the risk for recurrence often depends upon the particular risk factor(s) involved (Table 17-2). In general, women with a history of prior VTE associated with an idiopathic risk factor (i.e.,
VTE during previous pregnancy, oral contraceptives, or evidence of inherited thrombophilia) are at increased risk for recurrence of VTE compared to women with prior VTE that was associated with a nonrecurrent or transient risk factor (i.e., prolonged immobility or surgery).27,28 Additionally, those women with a family history of VTE in a first-degree relative (i.e., parent or sibling) before age 50 may also be at increased risk for VTE during pregnancy.27
VTE during previous pregnancy, oral contraceptives, or evidence of inherited thrombophilia) are at increased risk for recurrence of VTE compared to women with prior VTE that was associated with a nonrecurrent or transient risk factor (i.e., prolonged immobility or surgery).27,28 Additionally, those women with a family history of VTE in a first-degree relative (i.e., parent or sibling) before age 50 may also be at increased risk for VTE during pregnancy.27
Other risk factors, with or without underlying thrombophilia, contribute to thrombogenesis, again emphasizing that VTE is a multifactorial process. The risk factors, other than personal or family history of VTE or thrombophilia, that carry the greatest predisposition to VTE during pregnancy include increasing age, obesity, and mode of delivery, primarily Cesarean section.2,9,10,12,13,16,9,29,30,31,32 Increasing age and obesity may contribute to the risk of VTE secondary to disturbed blood flow, increase in clotting factors, or impaired fibrinolytic activity.16 The risk for VTE in the general population varies based upon type of surgical procedure as well as the presence of other comorbid conditions, with the greatest risk occurring in orthopedic surgery or surgery for malignancies. Surgical procedures, such as laparotomy, may present less risk for VTE because of reduced tissue damage from the surgical procedure and/or better mobilization following surgery.16 Although Cesarean section may be considered a relatively low-risk surgical procedure (i.e., early mobilization following delivery and short duration of surgery), it is believed to increase the risk for VTE two- to tenfold over vaginal delivery, possibly from endothelial or vascular damage during delivery.7,12 The majority of postpartum PE is most strongly associated with Cesarean section over other modes of delivery.13 Emergency Cesarean section is more hazardous than elective Cesarean section.2,33 Obesity, while an independent risk factor for VTE, also presents additional predisposition at the time of Cesarean section for VTE. These include a higher risk of blood loss from delivery, prolonged operating time, increased use of uterotonics, and postpartum wound infection or endometritis.30
The presence of multiple risk factors, with or without Cesarean section, increases the risk for VTE. These include operative vaginal delivery, major pelvic or abdominal surgery at the time of Cesarean section, higher parity, prolonged immobility, inflammatory process or active infection, dehydration, gross varicose veins, or chronic disease such as nephrotic syndrome, sickle cell disease, or heart disease.7,9,10,13,14,22,34,35 The presence of multiple risk factors, rather than a single risk factor, is probably of greater concern regarding the overall risk for VTE. Consensus is lacking as to which risk factors necessitate thromboprophylaxis.27,36,37,38 The decision to administer anticoagulants for either prophylaxis or treatment versus no pharmacologic treatment during the antpartum and postpartum periods is individualized based upon the physician’s assessment of the patient’s VTE history, thrombogenecity of the inherited thrombophilia, and presence of additional risk factors. In general, postpartum anticoagulant dosages should be equivalent or greater to the dosages prescribed during the antepartum period.27
Thrombophilias
Antithrombin Deficiency
AT deficiency arises from multiple possible mutations that result in either reduction in circulating AT III or decreased AT III activity. The deficiency promotes increased thrombosis because of reduction in or impaired removal of thrombin and activated factor X (Xa). Regulation of coagulation is further hindered by the deficiency in AT because the in-activation of factors XIIa, XIa, and IXa is blocked. Inheritance of AT deficiency is typically by autosomal dominance and almost always presents in the heterozygous state. Homozygous manifestation of AT deficiency is extremely rare and is usually lethal early in life through neonatal thrombosis.39 AT deficiency is the rarest thrombophilia.
Factor V Leiden Mutation
Factor V Leiden mutation (FVL) manifests functionally as resistance to activated protein C and involves a mutation in the factor V gene, rendering it resistant to cleavage by activated protein C. Activated protein C resistance (APCR) hinders the breakdown of factor Va, creating a hypercoagulable state as a result of increased generation of thrombin.9,11,23,25 APCR is the most common cause of VTE; more than 95 percent of APCR is caused by FVL.11 FVL is more prevalent in Western Europeans and Caucasians compared to a low incidence in those of African American or Asian descent.9,11,26




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree