Perinatal Infection
Patrick Duff
This chapter focuses on the principal bacterial, viral, and protozoan infections that pose a serious threat to the health and safety of the pregnant woman and her fetus or newborn. The review includes a discussion of group B streptococcal infection, cytomegalovirus infection, viral hepatitis, herpes simplex infection, human immunodeficiency virus infection, parvovirus infection, rubella, syphilis, toxoplasmosis, and varicella. The discussion highlights the clinical manifestations, adverse maternal and fetal consequences, diagnosis, and perinatal management of each infection.
Cytomegalovirus Infection
Cytomegalovirus (CMV) is a DNA virus that is a member of the herpes virus family. Like herpes simplex virus, CMV may remain latent in host cells after the initial infection. Recurrent infection is usually due to reactivation of an endogenous latent virus rather than reinfection with a new strain of virus.1
CMV is not highly contagious, and, therefore, close personal contact is required for infection to occur. Horizontal transmission may result from transplantation of an infected organ or transfusion of infected blood, sexual contact, or from contact with contaminated saliva or urine. Vertical transmission may occur as a result of transplacental infection, exposure to contaminated genital tract secretions during delivery, or breast-feeding. The incubation period of the virus ranges from 28 to 60 days.2
Among young children, the most important risk factor for infection is close contact with playmates. Most children who acquire CMV infection are asymptomatic.3 When clinical manifestations are present, they include malaise, fever, lymphadenopathy and hepatosplenomegaly. Similarly, most immunocompetent adults with CMV infection are asymptomatic or have only mild symptoms suggestive of a flu-like illness.
The diagnosis of CMV infection can be confirmed by isolation of virus in tissue culture. The highest concentration of virus typically is in urine, seminal fluid, saliva, and breast milk. Polymerase chain reaction methodology permits identification of viral antigen within 24 hours.1,2
Serologic methods also are helpful in establishing the diagnosis of CMV infection. In the acute phase of infection, IgM antibody is present in serum. IgM titers usually decline rapidly over a period of 30 to 60 days, but they can remain elevated for many months. There is no absolute IgG titer that clearly differentiates acute from recurrent infection. However, a fourfold or greater change in the IgG titer is consistent with recent acute infection. In addition, detecting “low acidity” IgG also is indicative of recent infection.
As a result of exposure to either young children or an infected sex partner, approximately 50% to 80% of adult women in the United States have serologic evidence of past CMV infection. Unfortunately, the presence of antibody is not perfectly protective against either reinfection or vertical transmission. Therefore, pregnant women with both recurrent and primary infection pose a risk to their fetus.4,5
Antepartum (congenital) infection poses the greatest risk to the fetus and results from hematogenous dissemination of virus across the placenta. Dissemination may occur with both primary and recurrent infection, but it is much more likely in the former setting. In women who acquire primary CMV infection during pregnancy, approximately 40% to 50% of the fetuses will be infected. The overall risk of congenital infection is greatest when maternal infection occurs in the third trimester, but the probability of severe fetal injury is highest when maternal infection develops in the first trimester.4,5
Approximately 5% to 15% of infants who develop congenital CMV infection as a result of primary maternal infection will be symptomatic at birth. The most common clinical manifestations of severe neonatal infection are hepatosplenomegaly, intracranial calcifications, jaundice,
growth restriction, microcephaly, chorioretinitis, hearing loss, thrombocytopenia, hyperbilirubinemia, and elevated serum transaminase concentrations. Approximately 30% of severely infected infants die; 80% of survivors have major morbidity such as mental retardation, ocular abnormalities, or sensorineural hearing loss. Approximately 85% to 90% of infants with congenital CMV infection are asymptomatic at birth, and 10% to 15% subsequently develop hearing loss, chorioretinitis, or dental defects within the first two years of life.1,4,5
growth restriction, microcephaly, chorioretinitis, hearing loss, thrombocytopenia, hyperbilirubinemia, and elevated serum transaminase concentrations. Approximately 30% of severely infected infants die; 80% of survivors have major morbidity such as mental retardation, ocular abnormalities, or sensorineural hearing loss. Approximately 85% to 90% of infants with congenital CMV infection are asymptomatic at birth, and 10% to 15% subsequently develop hearing loss, chorioretinitis, or dental defects within the first two years of life.1,4,5
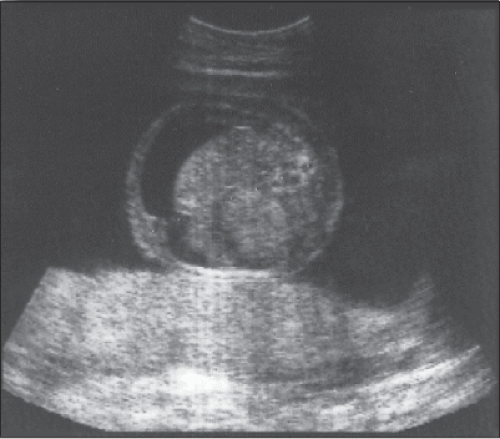
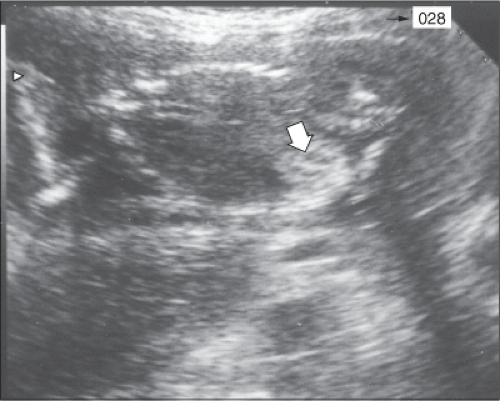 Figure 20-1 A longitudinal scan of the fetus shows echogenic bowel (arrowhead) characteristic of congenital CMV infection. |
Pregnant women who experience recurrent or reactivated CMV infection are much less likely to transmit infection to their fetus. When recurrent infection develops in pregnancy, approximately 5% to 10% of infants will become infected; however, none of these neonates are symptomatic at birth. The most common sequelae are hearing loss, visual deficits, and mild developmental delays.5
Perinatal infection can occur during delivery as a result of exposure to infected genital tract secretions. Infection also may occur as a result of breast-feeding. However, infants infected by one of these mechanisms rarely have any serious injury.
Identification of CMV in amniotic fluid by either culture or polymerase chain reaction (PCR) is the most sensitive and specific test for diagnosing congenital infection.6 However, mere identification of the virus does not necessarily delineate the severity of fetal injury. Fortunately, sonography is invaluable in providing information about severity of fetal impairment. The principal sonographic findings suggestive of serious fetal injury include microcephaly, ventriculomegaly, intracerebral calcification, fetal hydrops, growth restriction, and oligohydramnios. Less common findings include fetal heart block, intra-abdominal echodensities (Figure 20-1), meconium peritonitis, renal dysplasia, and isolated serous effusions (Figure 20-2).
Until recently, no consistently effective therapy for congenital CMV infection was available. However, in 2005, Nigro and colleagues,7 published an exciting report describing use of hyperimmune globulin as treatment and prophylaxis for congenital CMV infection. The authors performed a prospective cohort study at eight Italian medical centers. One hundred fifty-seven women had confirmed primary CMV infection. Of these, 148 were asymptomatic and were identified by routine serologic screening; eight had symptomatic viral infection, and one was identified because her fetus had abnormal ultrasound findings. Forty-five women had a primary infection more than 6 weeks before enrollment, underwent amniocentesis, and had CMV detected in amniotic fluid by PCR or culture. Thirty-one of these women received intravenous treatment with CMV-specific hyperimmune globulin (200 units, or mg, per kilogram of maternal body weight). Nine of the 31 received one or two additional infusions into either the amniotic fluid or umbilical cord because of persistent fetal abnormalities on ultrasonography. Fourteen women declined treatment; seven of them had infants who were acutely symptomatic at the time of delivery. In contrast, only one of the 31 treated women had an infant with clinical CMV disease at birth (adjusted odds ratio 0.02, p <.001).
In this investigation, 84 additional women did not undergo amniocentesis because their infection occurred within six weeks before enrollment, their gestational age was less than 20 weeks, or they declined amniocentesis. Thirty-seven of these women received 100 units of hyperimmune globulin per kilogram intravenously
every month until delivery, and 47 declined treatment. Of the treated women, six delivered infected infants as compared with 19 of the untreated women (adjusted odds ratio 0.32, p = 0.04). No adverse effects of hyperimmune globulin were noted in either group receiving immunotherapy.
every month until delivery, and 47 declined treatment. Of the treated women, six delivered infected infants as compared with 19 of the untreated women (adjusted odds ratio 0.32, p = 0.04). No adverse effects of hyperimmune globulin were noted in either group receiving immunotherapy.
This intriguing report had several shortcomings.8 The design of the study was neither randomized nor controlled. There are at least some biological reasons to question the remarkable success rates reported by the authors. Patients were not specifically stratified by presence or absence of major ultrasound abnormalities. Finally, the authors did not address the financial and logistic issues associated with screening large obstetric populations for CMV infection, triaging patients with inevitable false positive results, offering amniocentesis and targeted sonography to women who seroconvert, and then treating at risk patients with hyperimmune globulin. Nevertheless, the authors’ observations are extremely interesting and promising and offer the best available therapy for this dangerous perinatal infection.
Ideally, preventive measures should be employed to ensure that women do not contract CMV infection during pregnancy. One simple measure is using CMV-negative blood products when transfusing pregnant women or fetuses. In addition, women should be encouraged to use careful handwashing techniques after handling infant diapers and toys.1,8
Group B Streptococcal Infection
Streptococcus agalactiae is a gram-positive encapsulated bacterium that produces beta-hemolysis when grown on blood agar. On average, approximately 20% to 25% of pregnant women are colonized with group B streptococci in the lower genital tract and/or rectum. The group B Streptococcus is one of the most important causes of early-onset neonatal infection. The prevalence of neonatal group B streptococcal infection is approximately 0.5 per 1,000 live births, and approximately 10,000 cases of neonatal streptococcal septicemia occur each year in the United States.9
Approximately 80% to 85% of cases of neonatal group B streptococcal infection are early in onset, and these cases result almost exclusively from vertical transmission from a colonized mother to her infant. Early-onset infection typically presents as a severe pneumonia and/or overwhelming septicemia. In preterm infants, the mortality approaches 25%. In term infants, the mortality is lower, averaging approximately 5%.9
The major risk factors for early-onset infection include preterm labor, especially when complicated by preterm premature rupture of membranes; intrapartum maternal fever (due to chorioamnionitis); prolonged rupture of membranes (more than 18 hours); and previous delivery of an infected infant. Approximately 25% of pregnant women have at least one risk factor for early-onset group B streptococcal infection, and the presence of a risk factor dramatically affects the incidence of infection and the ultimate prognosis in infected neonates. For example, the neonatal attack rate in colonized patients is 40% to 50% when a risk factor is present and less than 5% in the absence of a risk factor. Moreover, in infected neonates, neonatal mortality approaches 30% to 35% when a maternal risk factor is present but is less than 5% when a risk factor is absent.9
At the present time, the gold standard for diagnosis of group B streptococcal infection is bacteriologic culture. The preferred medium is Todd-Hewitt broth or selective blood agar. The specimen for culture should be obtained from the lower vagina, perineum, and anus using a simple cotton swab.10 Bergeron and colleagues recently reported exceptionally favorable results with a new PCR assay for group B streptococci.11 In a series of 112 patients, the authors demonstrated a sensitivity of 97%, specificity of 100%, positive predictive value of 100%, and negative predictive value of 99%. This PCR assay now is commercially available and offers great promise as a rapid test for screening patients for group B streptococcal infection at the time that they are admitted to the labor and delivery unit. This test, and other nucleic acid amplification tests, are most effective when the laboratory specimen is allowed to incubate for 18 to 24 hours.10
The most effective strategy for prevention of early-onset neonatal group B streptococcal infection is based on guidelines published by the Centers for Disease Control and Prevention in 1996 and most recently modified in 2010.12 The new guidelines recommend culturing select patients admitted to the hospital for assessment of preterm labor and/or preterm premature rupture of membranes. In addition, all patients should be cultured at 35 to 37 weeks. Patients who test positive should receive intrapartum antibiotic prophylaxis with one of the regimens outlined in Table 20-1. Women who previously delivered a baby who had group B streptococcal infection, women known to be colonized earlier in pregnancy, and women with prior group B streptococcal bacteriuria should not be routinely cultured at 35 to 37 weeks. They should be considered colonized and treated with prophylactic antibiotics intrapartum.
Ideally, antibiotics should be administered at least 4 hours prior to delivery. DeCueto and associates recently demonstrated that the rate of neonatal group B streptococcal infection was reduced significantly when patients were treated for at least this period of time.13 Overall, if the guidelines outlined above are followed, the risk of neonatal infection should be reduced by approximately 80% in treated patients.14
Hepatitis
Hepatitis A
Hepatitis A, the second most common form of viral hepatitis in the United States, is caused by an RNA virus. The virus is transmitted by fecal–oral contact, and the incubation period varies from 15 to 50 days. Acute infections in children are usually asymptomatic; infections in adults are usually symptomatic. The diagnosis of acute infection is best confirmed by detection of IgM antibody specific for the hepatitis A virus. Hepatitis A does not cause a chronic carrier state. Perinatal transmission virtually never occurs, and, therefore, the infection does not pose a major risk to either the mother or the fetus unless the mother develops fulminant hepatitis and liver failure. Fortunately, this complication is extremely rare.15
Table 20.1 Intrapartum Prophylaxis for Group B Streptococcal Infection | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
Hepatitis A can be prevented by administration of an inactivated vaccine.16 Standard immune globulin also provides reasonably effective passive immunization for hepatitis A if it is given within two weeks of exposure, for example, in anticipation of travel to an area of the world where hepatitis A is endemic.
Hepatitis E
Hepatitis E is caused by an RNA virus, and the epidemiology of this infection is similar to that of hepatitis A. The disease is rare in the United States but is endemic in developing countries of the world. In these countries, maternal infection with hepatitis E has an alarmingly high mortality rate, in the range of 10% to 20%. This high mortality is due to the combined effect of hepatitis and poor nutrition, poor general health, and lack of access to modern medical care.17,18
Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally and by sexual contact. The virus also can be transmitted perinatally from an infected mother to her infant. Acute hepatitis B occurs in approximately 1 to 2 patients per 1,000 pregnancies in the United States; the chronic carrier state is present in 6 to 10 patients per 1,000 pregnancies.15
Approximately 90% of patients with hepatitis B mount an effective immunologic response to the virus and completely clear their infection. Less than 1% of infected patients develop fulminant hepatitis and die. Approximately 10% develop a chronic carrier state. The carrier state predisposes patients to severe chronic liver diseases such as chronic active or persistent hepatitis, cirrhosis, and hepatocellular carcinoma.15
The best method for diagnosing hepatitis B infection is serology. Table 20-2 demonstrates the possible results of serologic tests for hepatitis B virus. Clinicians should recognize that when the little e antigen is present in association with the surface antigen, viral replication is extensive and the patient is highly infectious.
Table 20.2 Serologic Diagnosis of Hepatitis B Infection | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Approximately 20% of pregnant women who are seropositive for hepatitis B surface antigen, but negative for the e antigen, will transmit infection to their neonates in the absence of intervention. Approximately 90% of mothers who are positive for both the surface antigen and e antigen will transmit infection.
Fortunately, excellent immunoprophylaxis for prevention of perinatal transmission of hepatitis B infection is now available. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. This passive immunization provides immediate protection against virus to which the infant is exposed during the birth process. Prior to discharge from the hospital, infants should begin the hepatitis B vaccination series. Active vaccination provides prolonged protection against hepatitis B. Infants delivered to mothers who are seronegative for hepatitis B require only the hepatitis B vaccine.15,21
Hepatitis D (Delta Virus Infection)
Hepatitis D is an RNA virus that depends upon coinfection with hepatitis B for replication. The epidemiology of hepatitis D is identical to that of hepatitis B. Patients who have both acute hepatitis B and hepatitis D are considered to have coinfection. These individuals typically clear both infections and have a very good long-term prognosis. Patients who have chronic hepatitis D infection superimposed upon chronic hepatitis B are considered to have a superinfection. These individuals are particularly likely to develop chronic liver disease.22,23 The diagnosis of hepatitis D can be established by identifying the delta antigen in liver tissue or serum. However, the most useful diagnostic tests are detection of IgM and/or IgG antibody in serum.
Hepatitis C
Hepatitis C is caused by an RNA virus. Infection may be transmitted parenterally, via sexual contact, and perinatally.24,25 Hepatitis C infection usually is asymptomatic. The diagnosis is confirmed by serologic testing. The initial screening test is an enzyme immunoassay (EIA), and the confirmatory test is a recombinant immunoblot assay (RIBA). The present generation of serologic tests does not consistently and precisely distinguish between IgM and IgG antibody.
In patients who have a low concentration of hepatitis C RNA and who do not have coexisting HIV infection, the risk of perinatal transmission of infection is low — less than 5%. In patients who have a high serum concentration of hepatitis C RNA and/or have HIV infection, the perinatal transmission rate may approach 25%. Several non-randomized, uncontrolled cohort studies (level II evidence) support the role for an elective Cesarean delivery prior to the onset of labor and ruptured membranes in select women who have a high titer of hepatitis C virus RNA. For women who have undetectable serum concentrations of viral RNA, vaginal delivery is a reasonable plan of management. In addition, breast-feeding is acceptable and does not pose a significant risk of transmission of infection to the neonate.26,27
Hepatitis G
Hepatitis G is caused by an RNA virus that is related to the hepatitis C virus. Hepatitis G is more common than hepatitis C, but, fortunately, it is much less virulent. Many patients who have hepatitis G are coinfected with hepatitis A, B, and C, and with HIV.28,29,30
Most patients with hepatitis G are asymptomatic. The diagnosis is established by detection of virus by PCR and by identification of antibody by enzyme-linked immunosorbent assay (ELISA). Hepatitis G can cause a chronic carrier state, and perinatal transmission has been documented. However, the clinical effect of infection in the mother and baby is minimal.28,29,30
Herpes Simplex Virus Infection
Herpes simplex virus (HSV) is a DNA virus that has two principal strains — HSV1 and HSV2. The former typically causes oral lesions, and the latter usually causes genital lesions. However, HSV1 can be responsible for ulcerated genital lesions, and HSV2 can be responsible for oral lesions. The infection is transmitted by intimate personal contact and is highly contagious.31
HSV2 is usually the organism of greatest concern to the perinatal and pediatric clinicians. HSV2 infections may be classified as primary, initial–non-primary, and recurrent, in accordance with the criteria listed in Table 20-3.
The characteristic lesion of HSV2 infection is a vesicle (Figure 20-3) that progresses, in sequence, to a pustule, then a shallow-based ulcer, then a crusted lesion. The duration of each of these lesions varies significantly, depending upon whether the infection is primary or recurrent, as noted in Table 20-4.
The most useful test for the clinical diagnosis of HSV infection is identification of the virus in the lesion by culture or PCR. When the lesion is in the vesicular stage, the frequency of viral isolation exceeds 90%. When the pustule is present, the frequency of viral isolation is
approximately 85%. When the ulcer is present, the frequency of viral isolation is approximately 70%. When the lesions have crusted, the frequency of viral isolation declines to only 25%.31
approximately 85%. When the ulcer is present, the frequency of viral isolation is approximately 70%. When the lesions have crusted, the frequency of viral isolation declines to only 25%.31
Table 20.3 Classification of Herpes Simplex Virus-2 Infections | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
HSV infection in pregnancy poses a risk to the fetus primarily at the time of delivery. Although the virus can be transmitted hematogenously across an intact placenta or transcervically across intact membranes, such transmission is extremely uncommon. The most likely mechanism of infection is exposure of the neonate to virus in the lower genital tract during the process of delivery. If a mother has a primary infection at the time of labor, and the infant delivers vaginally, the risk of infection is approximately 40%. In the era before the availability of acyclovir, at least half of these infants died, and most of the remainder had serious neurologic morbidity. If the mother has overt recurrent infection at the time of vaginal delivery, the risk of fetal infection is less than or equal to 5%. If the mother has a history of recurrent herpes but is simply asymptomatically shedding the virus at the time of delivery, the risk to the neonate is less than or equal to 1%.32,33,34,35
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree