Acute Renal Failure
Betsy B. Kennedy
Carol J. Harvey
George R. Saade
Acute renal failure (ARF), also referred to as acute kidney injury (AKI), broadly refers to a condition characterized by a relatively sudden and sustained decline in renal function. Criteria for ARF, described by the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group, include an abrupt reduction in kidney function, defined as an absolute increase in serum creatinine of more than 0.3 mg/dL or more than 25 micromoles/L, a 50 percent increase in serum creatinine, or oliguria, defined as less than 0.5 mL/kg/hr for more than 6 hours.1
The consequences of this dysfunction include failure of the kidneys to adequately excrete nitrogenous waste products, resulting in increased serum levels of protein metabolism derivatives (i.e., azotemia), an inability to maintain fluid and electrolyte balance, and increased risk of significant sequelae. The development of ARF in any patient increases the risk for death and is further increased if renal replacement therapy (e.g., dialysis) is needed.
Theoretically, rapid-onset ARF in a patient with no history of renal impairment is a reversible condition that does not always leave a patient with permanent impairment. However, the likelihood of recovery is dependent upon the type of ARF and its duration. To prevent progression of ARF requiring maintenance dialysis or a renal transplant, it is important to assess for ARF based on a high degree of suspicion, to quickly correct the underlying condition that is causing ARF, and to prevent further complications in the patient to enhance the chance for recovery.
This chapter addresses normal renal physiology, the impact of pregnancy on renal physiology, classification systems for ARF, common causes of ARF in pregnancy, and current trends in the management of ARF in pregnancy, including renal replacement therapies. Brief clinical case excerpts are presented to highlight significant differences between types of ARF.
Incidence of Arf
The exact incidence of ARF in pregnancy is difficult to determine as historically there have been no standard definitions of ARF in any population. Over the last 50 years the incidence in pregnancy has decreased in industrialized countries from 1 per 3,000 pregnancies to 1 per 15,000 to 20,000 pregnancies in women with no history of renal impairment.2,3 The decrease has been attributed to the reduction of septic abortions (secondary to the legalization of abortion in industrialized nations) and the increase in accessible prenatal care with a resultant decrease in maternal deaths.2,3 Prakash and colleagues in India recently reported a significant (p < 0.001) fall in the incidence of cortical necrosis related to ARF in pregnancy in a patient group from 1992 to 2002 compared to a similar group from 1982 to 1991. They concluded that the changing trends in obstetric ARF in their population were mainly related to a decrease in the number of septic abortions, puerperal sepsis, and maternal mortality.2 Although ARF occurs infrequently in the general pregnant population, it remains a common complication in critically ill patients and independently increases the risk for maternal mortality.4
The exact incidence of ARF and related mortality rates is elusive not only because of the prior use of non-standardized definitions of the disease, but also the lack of consistent use of International Classification of Disease (ICD) Clinical Modifications 9 and/or 10 codes for ARF, including diagnoses, types, and mortality secondary to the disease. Thus, epidemiologic study of ARF in various population groups and its outcomes is challenging. The incidence of ARF in all patients has been reported at 1 to 5 percent of hospital admissions, and mortality rates have ranged from 25 to 90 percent.
Similarly dismal has been the suggestion that there have been no measurable improvements in morbidity and mortality rates over the past two decades.5,6 In an attempt to counter this suggestion, two large retrospective
studies based on administrative databases reported that although the overall rate of ARF had increased, morbidity and mortality rates had decreased over time.7,8 Although the studies share the same limitations as other reports that rely upon extrapolated data from Medicare databases, state death certificates, and/or ICD-9 coding reports, a statistically significant improvement in outcome measures agrees with current clinical commentary on improved patient outcomes when evidence-based treatment plans are implemented. However, even when the improvement is accounted for, the outcomes of patients with ARF remain poor.9
studies based on administrative databases reported that although the overall rate of ARF had increased, morbidity and mortality rates had decreased over time.7,8 Although the studies share the same limitations as other reports that rely upon extrapolated data from Medicare databases, state death certificates, and/or ICD-9 coding reports, a statistically significant improvement in outcome measures agrees with current clinical commentary on improved patient outcomes when evidence-based treatment plans are implemented. However, even when the improvement is accounted for, the outcomes of patients with ARF remain poor.9
In light of current reports, it may be reasonable to say that the rate of renal insufficiency has increased in the general population across all age groups and that approximately 4 to 5 percent of non-pregnant hospitalized patients develop ARF, which may lead to further complications and death, frequently from infection and/or cardiopulmonary collapse. Although the rate has decreased over the past 15 to 20 years, 40 to 70 percent of all patients admitted to an intensive care unit (ICU) without a history of renal impairment continue to die from ARF.10 Morbidity and mortality rates for pregnant women who develop ARF are largely indeterminate, again related to the lack of a national database to follow the small number of pregnant patients admitted to ICUs. Additionally, the definition of ARF in pregnancy, like other specialties has only recently been developed.
Most cases of ARF in pregnancy occur in women with no previous renal disease. However, women with underlying chronic renal dysfunction (serum creatinine of 1.4 mg/dL or above) are at significantly increased risk for further loss of renal function during pregnancy.11 Approximately 40 percent of these women will have an added loss in renal function, and many will present with abrupt onset and progression.6 Thus the absence of ARF in pregnancy at any gestational age does not preclude the possibility that the woman may develop sudden-onset ARF and have a rapid deterioration of renal function.
ARF during pregnancy is rare, but when present, the concomitant risks pose clinical challenges for care providers. Because of associated mortality risks and potential for long-term morbidity, a multidisciplinary team of care providers that represents critical care, maternal-fetal medicine, obstetric critical care, and nephrology and neonatology specialties is recommended for clinical management.
Normal Kidney Function
Early identification and classification of ARF is commonly based on the interpretation of serum and urine laboratory tests that reflect kidney function. A brief review of renal anatomic and physiologic principles is presented, in order to better understand changes associated with ARF.
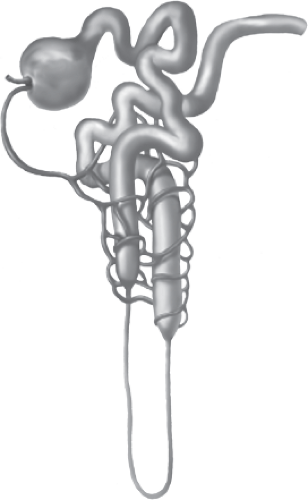
Normally, the renal/urinary system is composed of two kidneys, bilateral ureters, the urinary bladder, and the urethra. The functional unit of the kidney is the nephron, illustrated in Figure 13-1, with each adult kidney containing approximately 1 to 1.5 million nephrons. The nephron consists of a vascular and tubular component. Blood flows from the abdominal aorta into the renal arteries, the smaller renal arteries and arterioles, ending in the afferent arteriole, and ultimately in the glomerulus. The highly permeable capillaries in the glomerulus reform into the efferent arteriole, which then branches into the peritubular capillaries and vasa recta. The peritubular capillaries and vasa recta communicate with the renal tubules, to facilitate movement of water and solutes (secretion and reabsorption) between the plasma (peritubular capillaries and vasa recta), and filtrate (renal tubules).
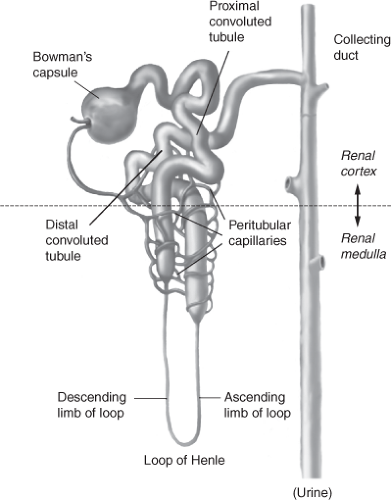
Bowman’s capsule surrounds the glomerulus and is considered the starting point of the tubule that participates in secretion and reabsorption (Fig. 13-2). The tubule is a continuous structure, divided into the proximal convoluted straight tubule, the descending limb, the ascending limb (together referred to as the loop of Henle), the distal convoluted tubule, and the cortical and medullary collecting ducts. The tubule is responsible for reabsorption of water, electrolytes, and other substances back into the blood of the peritubular capillaries, and into the systemic circulation (Fig. 13-3). The tubule exits into the collecting ducts, creating urine, which is drained into the ureters and stored in the bladder.
Nephrons produce urine via three processes: tubular reabsorption, tubular secretion, and glomerular
filtration.12 The kidneys receive up to 25 percent of cardiac output per minute, resulting in a continuous filtration of fluid from the glomerular capillary bed into Bowman’s capsule. The glomerular filtration rate (GFR) affects the amount of urine produced, waste products excreted, electrolyte balance, fluid balance, and acid base balance. The kidneys are usually able to autoregulate to maintain the GFR despite variations in arterial blood pressure and renal perfusion pressure. Even wide changes in arterial blood pressure, within the normal limits of 70 mmHg to 160 mmHg, have little to no effect on GFR. The juxtaglomerular apparatus (JGA), a group of cells positioned where the distal convoluted tubule of each nephron meets the angle of the afferent and efferent arterioles, controls tubuloglomerular feedback (renal autoregulation). Changes in the tubular fluid volume and electrolytes are sensed by the macula densa and relayed to the JGA. The JGA stimulates afferent arteriole vasodilation or constriction, affecting blood flow and glomerular capillary bed hydrostatic pressure, in order to maintain GFR.
filtration.12 The kidneys receive up to 25 percent of cardiac output per minute, resulting in a continuous filtration of fluid from the glomerular capillary bed into Bowman’s capsule. The glomerular filtration rate (GFR) affects the amount of urine produced, waste products excreted, electrolyte balance, fluid balance, and acid base balance. The kidneys are usually able to autoregulate to maintain the GFR despite variations in arterial blood pressure and renal perfusion pressure. Even wide changes in arterial blood pressure, within the normal limits of 70 mmHg to 160 mmHg, have little to no effect on GFR. The juxtaglomerular apparatus (JGA), a group of cells positioned where the distal convoluted tubule of each nephron meets the angle of the afferent and efferent arterioles, controls tubuloglomerular feedback (renal autoregulation). Changes in the tubular fluid volume and electrolytes are sensed by the macula densa and relayed to the JGA. The JGA stimulates afferent arteriole vasodilation or constriction, affecting blood flow and glomerular capillary bed hydrostatic pressure, in order to maintain GFR.
 Figure 13-2 Bowman’s capsule surrounds the glomerulus and is considered the starting point of the tubule that participates in secretion and reabsorption. |
Hydration status causes the kidneys to alter the amount of urine output. Fluid volume excess causes decreased tubular reabsorption of filtrate, resulting in large amounts of dilute urine. Fluid volume deficit causes maximal reabsorption of tubular filtrate, resulting in a small amount of concentrated urine. The kidneys are responsible for excretion of metabolic waste products including, but not limited to, urea, creatinine, uric acid, bilirubin, and metabolic acid.
While the respiratory system is primarily responsible for the regulation of acid–base balance, excreting large amounts of carbon dioxide each day, the kidneys excrete fixed acids (acid anion and associated hydrogen ion), for which there is no other means of removal. The kidneys are also responsible for reabsorption of filtered bicarbonate, the most important buffer for fixed acids.
In summary, select essential functions of the kidneys are: maintenance of intravascular volume; regulation of water balance, electrolyte balance, and plasma osmolality; regulation of acid–base balance in association with the respiratory and buffer systems; excretion of the end-products of metabolism and some exogenous substances (drugs); and participation in blood pressure regulation.13
Effects of Pregnancy on Kidney Function
During pregnancy, GFR increases approximately 40 to 65 percent, and renal blood flow increases even more, to approximately 50 to 85 percent.6,14 This increased perfusion leads to a 50 percent increase in the GFR and, in combination with the increase in renal plasma flow, accounts for more efficient clearance of several substances from
the blood, including creatinine and urea. This leads to lower serum levels of both substances. A normal creatinine level in pregnancy is 0.46 mg/dL, whereas a normal blood urea nitrogen (BUN) is 8.24 mg/dL. There is also a physiologic decrease in plasma osmolality as early as the first trimester. Sodium and water are retained during the course of pregnancy, with approximately 950 mEq of sodium and 6 to 8 liters of water accumulated. Thus, it can be said that pregnancy is a state of “super” or augmented renal clearance, impacting the function of the renal system and diagnostic criteria for ARF.15
the blood, including creatinine and urea. This leads to lower serum levels of both substances. A normal creatinine level in pregnancy is 0.46 mg/dL, whereas a normal blood urea nitrogen (BUN) is 8.24 mg/dL. There is also a physiologic decrease in plasma osmolality as early as the first trimester. Sodium and water are retained during the course of pregnancy, with approximately 950 mEq of sodium and 6 to 8 liters of water accumulated. Thus, it can be said that pregnancy is a state of “super” or augmented renal clearance, impacting the function of the renal system and diagnostic criteria for ARF.15
Table 13.1 Normal Alterations of Renal Function in Pregnancy | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Specific physiologic and anatomic changes associated with pregnancy that affect the renal system are described in Table 13-1. Hydronephrosis and hydroureter, which normally occur in pregnancy, affect renal function as evidenced by adjustments in laboratory parameter reference ranges.
Assessment of Kidney Function
Evaluation of kidney function includes serum and urine laboratory analyses and, commonly, renal imaging studies. Patient condition and assessment findings determine the level of diagnostic testing.
One of the fundamental components of assessment is urine output in milliliters per hour (mL/hr). Precise measurement is important in the diagnosis of ARF, as the volume of urine output plays a role in the prediction of patient morbidity and mortality rates. Oliguric ARF (less than 400 mL/24 hr) has a worse prognosis compared to nonoliguric ARF.5
A comparison of normal non-pregnant and pregnant values for select serum and urine indices is presented in Table 13-2.
Definition and Classifications of Arf
Patients at Risk
Patients at highest risk for ARF include those with co-morbidities such as diabetes mellitus, preexisting renal insufficiency, cardiac failure, or sepsis.16 The kidneys are dependent upon adequate oxygen delivery and consumption to maintain metabolic efficiency and avoid ischemic or hypoxemic injury. Physiologic stress produces a series of orchestrated measures to best manage overall survival. Predetermined measures will eventually result in the interruption of oxygen delivery to select organ systems that are not critical for survival. Common “non-critical” systems include integumentary, gastrointestinal, reproductive, and renal. Adaptive responses shunt arterial blood from these systems to more critical organs involved in survival: the heart, brain, and adrenal glands. Hence, all patients in unstable physiologic states are at risk for developing ARF. Acute clinical conditions associated with development of renal failure in hospitalized patients are extensive. These conditions include but are not limited to sepsis, septic shock, hypotension, hemorrhage, volume depletion, cardiac/vascular surgery, organ transplantation surgery, abdominal compartment syndrome, and mechanical ventilation.16,17
Risk factors for ARF in pregnant patients are the same as those for the general population. Risk is also affected by the patient’s age, physiologic status prior to hospital admission, specific etiology of the renal insult, and timing of identification and treatment. Risk factors often present in cases of ARF during pregnancy include hypertension, disseminated intravascular coagulation (DIC), infection, hypovolemia, and obstruction by the gravid uterus.6 Hypertensive complications that increase risk for ARF are most commonly preeclampsia-eclampsia, principally with co-morbidities such as placental abruption, pulmonary edema, or hemorrhage. The exact rate of ARF in preeclampsia
remains debatable, but current data suggest that 1.5 to 2 percent of women with preeclampsia develop ARF, and in patients with HELLP syndrome (see Chapter 7), the rate increases to greater than 7 percent.6 Causes of ARF in pregnancy and the most common times of onset are presented in Table 13-3.
remains debatable, but current data suggest that 1.5 to 2 percent of women with preeclampsia develop ARF, and in patients with HELLP syndrome (see Chapter 7), the rate increases to greater than 7 percent.6 Causes of ARF in pregnancy and the most common times of onset are presented in Table 13-3.
Table 13.2 Normal Laboratory Values in the Pregnant and Non-Pregnant Woman | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 13.3 Causes of ARF/AKI in Early Pregnancy, Late Pregnancy, and Postpartum | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The Rifle Criteria
Until recently, there was no agreement on an objective and measurable definition of ARF, which has hindered the investigation of the incidence and subsequent morbidity and mortality in patients with renal failure. In 2004, the
Acute Dialysis Quality Initiative (ADQI) group convened an International Consensus Conference of experts in the field and agreed upon a definition of ARF. Furthermore, the group created the “RIFLE” classification system based on changes from the patient’s baseline either in serum creatinine level or GFR, urine output, or both.18 The purpose of RIFLE, which is the acronym for Risk, Injury, Failure, Loss, and End-stage kidney disease (ESKD), is to classify patients at separate risk for development of ARF.19 The criteria are described in Table 13-4. A component of the RIFLE system is the use of urine output as a predictor of renal failure. The categorization of anuric, oliguric, nonoliguric, or polyuric urine output levels are defined in Table 13-5.
Acute Dialysis Quality Initiative (ADQI) group convened an International Consensus Conference of experts in the field and agreed upon a definition of ARF. Furthermore, the group created the “RIFLE” classification system based on changes from the patient’s baseline either in serum creatinine level or GFR, urine output, or both.18 The purpose of RIFLE, which is the acronym for Risk, Injury, Failure, Loss, and End-stage kidney disease (ESKD), is to classify patients at separate risk for development of ARF.19 The criteria are described in Table 13-4. A component of the RIFLE system is the use of urine output as a predictor of renal failure. The categorization of anuric, oliguric, nonoliguric, or polyuric urine output levels are defined in Table 13-5.
Table 13.4 RIFLE Criteria to Determine Risk for ARF | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
Classification
ARF can be classified as one of three general etiologic types: prerenal (hypoperfusion), intrinsic (intrarenal), or postrenal (obstructive) failure, depending upon the anatomic location of the problem.
Table 13.5 Definitions and Categories of Urine Output | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Prerenal failure is the result of disruption of oxygen and nutrient transport to the kidney. In pregnancy, the cause of decreased transport is frequently decreased cardiac output secondary to hemorrhage, hypovolemia, or hypotension. The primary etiology may be postpartum hemorrhage, septic shock, placental abruption, ruptured ectopic pregnancy, or DIC. If the kidneys are not perfused and oxygen and nutrient delivery restored, nephrons will become ischemic, which results in alteration of renal function based on the number of nephrons damaged. This type of renal failure is classified as intrinsic failure. Acute tubular necrosis (ATN) is one type of intrinsic failure. Postrenal failure, also termed obstructive renal failure, is caused by the obstruction of urine flow at any location. Frequently associated with hydronephrosis related to engorgement of the kidneys with urine, postrenal failure may cause nephron damage if left undiagnosed and/or untreated.
Each of the three types of renal failure has associated etiologies, history and physical assessment findings, and laboratory determinants. It is paramount for care providers to quickly identify the insult, correct the problem, reperfuse the kidneys, and provide supportive measures for the patient until recovery occurs.
Prerenal Failure
Prerenal azotemia is the most common form of ARF and results from an insult that occurs before blood reaches the kidneys. The kidneys receive approximately 20 to 25 percent of cardiac output per minute. When cardiac output is adequate, a mean arterial pressure (MAP) greater than 70 mmHg should maintain adequate renal perfusion. During periods of hypovolemia or decreased cardiac output from other causes, there is evidence that patients with a MAP less than 65 mmHg have an increased risk for ARF.20 Any condition that decreases cardiac output or limits systemic perfusion pressure, such as decreased intravascular volume and decreased vascular tone, may lead
to hypoperfusion of the kidneys. Initially, the normal kidneys adapt by afferent arteriole dilation and efferent arteriole constriction to maintain normal GFR (autoregulation), and by renin release. Renin activates a cascade of events that results in peripheral vasoconstriction, increased water reabsorption, and increased serum BUN concentration. Although the structure of the kidneys is normal, the glomeruli eventually become unable to filter blood secondary to reduction in blood flow. Glucose and oxygen delivery to the tubular cells is decreased, and there is retention of metabolic wastes. The outcome is decreased adenosine triphosphate (ATP) synthesis in renal tubular cells. Many tubular processes are ATP-dependent, so numerous dysfunctions occur as a result of inadequate oxygen, glucose, and ATP. Elevated serum concentration of the nitrogenous waste products eventually occurs, and the kidneys progress to failure if the source is not identified and treated.
to hypoperfusion of the kidneys. Initially, the normal kidneys adapt by afferent arteriole dilation and efferent arteriole constriction to maintain normal GFR (autoregulation), and by renin release. Renin activates a cascade of events that results in peripheral vasoconstriction, increased water reabsorption, and increased serum BUN concentration. Although the structure of the kidneys is normal, the glomeruli eventually become unable to filter blood secondary to reduction in blood flow. Glucose and oxygen delivery to the tubular cells is decreased, and there is retention of metabolic wastes. The outcome is decreased adenosine triphosphate (ATP) synthesis in renal tubular cells. Many tubular processes are ATP-dependent, so numerous dysfunctions occur as a result of inadequate oxygen, glucose, and ATP. Elevated serum concentration of the nitrogenous waste products eventually occurs, and the kidneys progress to failure if the source is not identified and treated.
The adaptive response of functioning kidneys with intact nephrons to decreased oxygen and nutrient delivery includes release and activation of angiotensin II, aldosterone, and antidiuretic hormone (ADH). These produce increased reabsorption of sodium (Na+) and urea in nephrons. The increased level of serum Na+ results in increased intravascular volume and causes decreased urine output and, commonly, oliguria. This results in an increased concentration of urine (increased urine osmolality) and a decrease in urine Na+.
With timely identification and rectification of the underlying cause to reestablish systemic and therefore renal perfusion, the condition can be reversible. Treatment needs to be based on the etiology of the prerenal problem. Fluid administration with intravenous normal saline solution (0.9% NaCl), and/or blood transfusion in cases of hemorrhage or anemia, is most often a cornerstone of initial treatment of prerenal failure. If hypoperfusion persists and is not recognized or ineffectively managed, the protective mechanisms of the kidneys become depleted. The resulting ischemic damage may be permanent and lead to intrinsic failure (e.g., ATN). The amount of damage is dependent on the duration of the insult and the baseline health of the kidneys at the time of insult. Etiologies of prerenal failure can be found in Table 13-6.
Table 13.6 Etiologies of Prerenal Failure |
|---|