Upper Urinary Tract
John S. Wiener
Departments of Surgery and Pediatrics, Duke University, Section of Pediatric Urology, Duke University Medical Center, Durham, North Carolina 27710.
The upper urinary tract has the sole function of transporting urine from its origin (the nephrons within the kidney) to its storage organ (the bladder). Alteration of this transport of urine can be congenital or acquired. Maldevelopment of the upper urinary tract is relatively common and results in numerous congenital anomalies that are increasingly being detected prior to birth. Acquired diseases of the upper urinary tract are less common in children than in adults, but include urolithiasis and neoplasia. Manifestation of upper urinary tract pathology is usually related to obstruction and may be silent or painful, can be detrimental to the kidney and its multiple functions, and may promote infection secondary to stasis of urine. Infection of the urinary tract is typically localized to the bladder (cystitis) or renal parenchyma (pyelonephritis); the upper urinary tract may act as a conduit, allowing bacteria to ascend from the former to the latter. Retrograde flow of urine from the bladder into the upper urinary tract (vesicoureteral reflux), thus, can increase the likelihood of pyelonephritis.
ANATOMY OF THE UPPER URINARY TRACT
The upper urinary tract begins where the collecting ducts of the nephrons within each renal pyramid coalesce at a papilla and empty into a calyx. There are typically eight or nine calyces in each kidney, and these are usually grouped in sets in the upper, mid, and lower segments of the kidney (1). The renal pelvis fills most of the renal sinus in the center of the kidney and funnels medially and downward into the ureter across the ureteropelvic junction. The normal renal pelvis is usually wholly contained within the renal sinus, but can extend medially beyond the renal contour (termed an extrarenal pelvis.)
The ureter is intimately related to the peritoneum, psoas major muscle, and numerous blood vessels and nerves as it courses through the retroperitoneum from the ureteropelvic junction to the ureterovesical junction. The ureter contains a rich vascular plexus fed from multiple sources, including the renal artery, aorta, gonadal arteries, iliac arteries, and superior and inferior vesical arteries (1). Venous and lymphatic drainage are similar. Nervous input comes from multiple plexuses, including renal, aortic, superior hypogastric, and pelvic. Visceral pain caused by distention or irritation of the ureter is referred to somatic distribution along corresponding spinal segments and may manifest as pain in the flank, abdomen, groin, or scrotum and labia (2,3). Surgical anatomy of the ureter is simply divided into upper ureter (renal pelvis to upper sacral border), middle ureter (across the sacroiliac bony landmarks), and lower ureter (lower sacral border to bladder). The ureter passes through the muscularis layer of the bladder in an oblique tunnel normally of sufficient length to prevent retrograde flow of urine from the bladder back into the ureter. The ureter opens into the bladder at one of the lateral corners of the trigone—the triangular-shaped base of the bladder defined by the points of the two ureteral orifices and the internal urethral orifice.
PHYSIOLOGY OF THE UPPER URINARY TRACT
The muscles of the ureter contract in response to stretch from urine filling, and these contractions transport boluses of urine from the kidney to the bladder. This peristalsis can be seen radiographically on intravenous pyelograms or computed tomography (CT) scans as distinct small boluses of contrast-containing urine within the ureter. Complete opacification of the entire length of the ureter on such studies is not normal and would suggest distal obstruction. Ureteral peristalsis originates with electrical activity in pacemaker cells found in the calyces and is conducted distally via junctions between smooth muscle cells (4). Resting ureteral pressure is only 0 to 5 cm H2O and rises to 20 to 80 cm H2O with contraction. This is usually
higher than intravesical bladder pressure in the normal state, allowing passage of the urine bolus into the bladder (1,3,5). Obstruction of the flow of urine dilates the walls of the ureter, preventing adequate coaptation and propulsion of the urine distally. This, in turn, stretches the renal pelvis and capsule and may be manifested clinically as renal colic.
higher than intravesical bladder pressure in the normal state, allowing passage of the urine bolus into the bladder (1,3,5). Obstruction of the flow of urine dilates the walls of the ureter, preventing adequate coaptation and propulsion of the urine distally. This, in turn, stretches the renal pelvis and capsule and may be manifested clinically as renal colic.
EMBRYOLOGY OF THE UPPER URINARY TRACT
At the end of the fourth week of embryonic life, the ureteral bud begins as an outgrowth from the mesonephric (wolffian) duct near its insertion into the cloaca. The ureteral bud then grows cephalad until it encounters the metanephros (Fig. 96-1). A complex interplay between the distal ureteral bud and metanephric blastema is responsible for branching of the ureteral bud and its induction of renal development. A multitude of genes have been linked to this process of nephrogenesis, and genetic alterations can result in congenital anomalies of the ureters and kidneys (6,7). Ectopic branching of the ureteral bud can impact the metanephros ectopically, resulting in renal maldevelopment encountered in some congenital upper urinary tract malformations, such as obstructive uropathies and vesicoureteral reflux. The normal ureteral bud will eventually branch 15 times as it grows radially into the metanephros, forming the renal pelvis, infundibula, and calyces (1). Distally, the ureteral bud and mesonephric duct are incorporated into the cloaca to form the trigone of the bladder and intramural portion of the ureter. The ureteral orifice migrates cranially and laterally, whereas the mesonephric ducts move distally and medially toward the bladder neck and urethra. The distal end of the latter structure becomes the epididymis, vas deferens, seminal vesicle, and ejaculatory duct in males and Gartner’s ducts on the anterior vaginal wall in females.
The ureteral bud is initially patent, but becomes obliterated in the sixth week. Two weeks later, recanalization begins in the midureter and extends caudally and cranially so the entire ureter should be patent again by the ninth or tenth week. Timing is critical because urine production commences by the eight to ninth week and will comprise more than 90% of the amniotic fluid by the early second trimester. Delayed recanalization at either end of the ureter can lead to obstruction and altered renal development and may explain why the two most common sites of congenital obstruction are at the ureteropelvic junction (UPJ) and ureterovesical junction (UVJ), respectively. Abnormal early branching of the ureteral bud is fairly common and leads to duplication anomalies of the upper urinary tract. It is estimated that 1 in 125 individuals have partial ureteral duplication (two ureters joining above the bladder with only a single distal ureter entering the bladder), and 1 in 500 have complete ureteral duplication (two separate ureters with separate orifices within the bladder) (8). It should be noted that this does not imply supranumerary kidneys, but merely two separate pelvises and ureters in one kidney on one or both sides. In complete duplication, the ureters cross so the upper pole ureter inserts medially and caudally, and the lower ureteral orifice is more lateral and cranial. The upper pole ureter may insert too far distally (termed ectopic) and be obstructed by its opening into the bladder neck, urethra, or mesonephric duct remnants (the reproductive ductal structures in males or Gartner’s duct in females). In rare cases in females with complete duplication, continuous lifelong urinary incontinence can occur due to an insertion of the upper pole ureter into the urethra or vagina. The lower pole ureter in completely duplicated systems may reflux due to insufficient muscular backing of the intramural ureter in its far lateral position.
PATHOLOGY OF THE UPPER URINARY TRACT
Pathology of the upper urinary tract is easily classified. Most pathology is due to obstruction, and most obstructive uropathies in childhood are congenital. Obstruction during fetal development of the urinary tract may alter development of the ureter and kidney, and compromise ultimate renal function. Acquired obstruction is unusual in childhood, but may be related to iatrogenic causes, neoplasms, or urolithiasis. Primary neoplasms of the ureter and renal collecting system in children are exceedingly rare. Finally, vesicoureteral reflux is a pathologic entity of the upper urinary tract that facilitates bacterial ascent up into the kidney and can result in recurrent pyelonephritis. In rare cases, reflux and obstruction can be present in the same ureter.
OBSTRUCTIVE UROPATHY
Pathophysiology
Obstruction of the upper urinary tract is usually partial, but can be complete. Complete obstruction destroys renal function eventually; partial obstruction may impair function, but the degree of impairment is dependent on timing of its onset (during prenatal development), chronicity, and degree of obstruction. Most of our knowledge regarding the effects of obstruction
stems from research focused on acute obstruction in postnatal models. The classic model of acute unilateral obstruction demonstrates a triphasic response: ureteral pressure and renal blood flow initially increase simultaneously, blood flow then decreases while ureteral pressure remains elevated, and finally both parameters decline (9). As obstruction becomes chronic, the decreased blood flow leads to irreversible declines in glomerular filtration rates and other components of renal function (10,11). Early studies of unilateral ureteral obstuction in a fetal lamb model found that obstruction in the first half of gestation resulted in renal dysplasia, whereas obstruction in the latter half of gestation only caused dilation of the pelvis and calyces (pelvicaliectasis) with preservation of histologic appearance (12). In the latter group, the degree of parenchymal atrophy was proportional to the period of obstruction. In other fetal models, it appears that increased pressure in the renal pelvis may not alter renal development; however, reduction of renal blood flow is likely deleterious (10). The fetal ureter may be able to undergo a greater degree of dilation and elongation in response to obstruction than the adult ureter, and this may dampen the pressure effects of obstruction (11). Further complexity in assessing the effects of fetal obstruction exists because normal and hydronephrotic kidneys may function similarly at normal rates of urine production, but diuresis may knock the partially obstructed kidney out of equilibrium (10). What is clear is that higher degrees and longer periods of obstruction are more deleterious to the kidney and that early fetal obstruction can affect renal development.
stems from research focused on acute obstruction in postnatal models. The classic model of acute unilateral obstruction demonstrates a triphasic response: ureteral pressure and renal blood flow initially increase simultaneously, blood flow then decreases while ureteral pressure remains elevated, and finally both parameters decline (9). As obstruction becomes chronic, the decreased blood flow leads to irreversible declines in glomerular filtration rates and other components of renal function (10,11). Early studies of unilateral ureteral obstuction in a fetal lamb model found that obstruction in the first half of gestation resulted in renal dysplasia, whereas obstruction in the latter half of gestation only caused dilation of the pelvis and calyces (pelvicaliectasis) with preservation of histologic appearance (12). In the latter group, the degree of parenchymal atrophy was proportional to the period of obstruction. In other fetal models, it appears that increased pressure in the renal pelvis may not alter renal development; however, reduction of renal blood flow is likely deleterious (10). The fetal ureter may be able to undergo a greater degree of dilation and elongation in response to obstruction than the adult ureter, and this may dampen the pressure effects of obstruction (11). Further complexity in assessing the effects of fetal obstruction exists because normal and hydronephrotic kidneys may function similarly at normal rates of urine production, but diuresis may knock the partially obstructed kidney out of equilibrium (10). What is clear is that higher degrees and longer periods of obstruction are more deleterious to the kidney and that early fetal obstruction can affect renal development.
Prenatal Diagnosis
A generation ago, most uropathies presented with urinary tract infection (UTI) or a palpable mass on physical examination. Hydronephrosis was previously considered the most common cause of abdominal masses in neonates (13). The advent of routine prenatal sonography beginning in the mid-1980s has dramatically changed the presentation of upper urinary tract anomalies in children, so that today abnormal prenatal sonogram is the most common presentation of uropathies (14). Urinary tract dilation is the most common anomaly seen on prenatal sonography, detected in as many as 1% of fetuses, and may represent a significant urologic problem in 20% of those or in 1 out of 500 fetuses (15,16). A commonly accepted threshold for abnormal renal pelvic dilation is an anteroposterior (AP) renal pelvic diameter of 4 mm or greater at less than 33 weeks’ gestation and 7 mm or greater beginning at 33 weeks’ gestation (17). Additional signs of uropathy on sonography include dilation of the calyces, ureters, and/or bladder; decreased amniotic fluid volume; and abnormal renal parenchyma (e.g., cysts, hyperechogenicity, or cortical thinning). Other abnormalities, including renal duplication and obstructing ureteroceles, can also be seen. Oligohydramnios can be an ominous finding because it may be associated with pulmonary hypoplasia as well as renal insufficiency.
The bladder is visible in the tenth week of gestation, and the kidneys may be noted by 12 to 13 weeks. Urinary tract anomalies have been detected as early as this point. The differential diagnosis of fetal hydronephrosis includes UPJ obstruction, distal ureteral obstruction (due to primary obstructive megaureter, ureteral ectopia, and ureteroceles), multicystic dysplastic kidneys, posterior urethral valves, prune belly syndrome, and vesicoureteral reflux (Table 96-1). Nephropathies unrelated to the upper urinary tract (polycystic kidney disease and renal agenesis) also can be detected (18).
Initially, it was believed that all fetuses with hydronephrosis had obstructive uropathies that required intervention (14). However, the majority of cases of prenatal hydronephrosis have been found to be clinically insignificant (19). Most are unilateral renal pelvic dilation that were the result of transient partial prenatal obstruction and will improve with age. Some cases of severe hydronephrosis will show normal renal function initially, but develop worsening obstruction over time, necessitating surgical correction. Other kidneys suffer damaging effects of obstruction in utero and present with irreversibly diminished function at birth. Determining what constitutes true obstruction remains a subject of great debate. It is evident that obstructive uropathies are dynamic, possibly due to continued development of the distal and proximal ends of the ureter during the fetal life and infancy. Maternal progesterone may also play a role as a smooth muscle relaxant. In cases of reflux, hydronephrosis may exist transiently or continuously due to retrograde filling of the upper urinary tract in the absence of obstruction and does not correlate well with the grade of reflux.
TABLE 96-1 Uropathies Detectable by Prenatal Sonography | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Prenatal Intervention
The detection of potentially lethal obstructive lesions of the upper urinary tract by prenatal sonography has provided the opportunity to correct such lesions in utero. Obstruction can be relieved by open fetal surgery, shunt placement between dilated portion of the urinary tract and the amniotic space, and even therapeutic fetoscopic procedures (18). Vesicoamniotic shunting has been used most extensively. These interventions in cases of bilateral hydronephrosis with oligohydramnios have been demonstrated to improve survival based on pulmonary outcomes, but have yet to demonstrate any prevention of renal insufficiency (20). These procedures carry a complication rate as high as 45% and include fetal demise (21). Therefore, fetal intervention remains highly controversial and is reserved for highly selected cases. Indications include persistent or progressive severe obstruction of both kidneys or a solitary kidney without overt signs of dysplasia, the presence of oligohydramnios, and favorable fetal urine functional indices (based on biochemical measurements) in an otherwise healthy fetus (18). In practice, these interventions are performed only in cases of bladder outlet obstruction (primarily posterior urethral valves) and have little to no role in obstruction of the upper urinary tract.
The detection of prenatal hydronephrosis should have little impact on obstetric care. Unilateral cases, even if they result in total loss of function in that renal unit, will not affect survival or longevity. A follow-up second or third trimester scan is helpful to see if the process is worsening or has resolved. Early delivery is not indicated because pulmonary maturation is of paramount concern. In bilateral cases, more frequent scanning, usually every 4 weeks, is indicated to monitor changes in amniotic fluid volume. If oligohydramnios is also present, then early delivery may be considered after 32 weeks or when fetal lung maturity is confirmed by lecithin-to-sphingomyelin amniotic fluid ratio.
Postnatal Diagnosis and Management
The urgency of postnatal workup is somewhat dependent on the severity of the prenatal findings. Unilateral hydronephrosis will not affect survival in the presence of a normal contralateral unit; whereas, bilateral severe pathology could potentially progress to renal failure. Unfortunately, there are few, if any, signs or symptoms to differentiate those with and without significant pathology. For this reason, a postnatal evaluation is indicated for all newborns who meet the definition of hydronephrosis based on renal pelvic AP diameter measurements (greater than 4 mm prior to 33 weeks or greater than 7 mm at or after 33 weeks) or who have ureteral dilation, bladder distension, and/or oligohydramnios.
Postnatal evaluation consists of sonography and voiding cystourethrogram (VCUG). The latter study is performed to exclude vesicoureteral reflux, urethral obstruction (primarily posterior urethral valves in males), or the presence of intravesical lesions (primarily ureteroceles). VCUG using standard constrast with fluoroscopy is preferable over radionuclide VCUG for the initial study due to superior anatomic detail. This study can be performed at any time, and we prefer prior to discharge from the nursery at our institution. In males without concern for urethral obstruction and in all females, it can be delayed, but the child should be assumed to have vesicoureteral reflux until proven otherwise and be placed on antibiotic prophylaxis (amoxicillin 15 mg per kg once daily). Some have argued that VCUG is an unnecessarily invasive procedure to perform in all neonates with a history of prenatal hydronephrosis, but it is not possible to exclude reflux by sonography alone. As many as 27% of those found to have reflux demonstrate no postnatal hydronephrosis, and reflux in the neonatal period is more common in males (22). Reflux is also found more frequently in the contralateral ureter in cases of renal agenesis, UPJ obstruction, and multicystic dysplastic kidney (18).
Postnatal sonography should include imaging of both kidneys, ureters, and bladder to fully evaluate both the upper and lower urinary tract. The timing of such intervention, however, remains controversial. Obstructive uropathy has been reportedly missed on sonography performed
in the first 48 hours of life because perinatal dehydration may prevent adequate distension of the urinary tract to detect hydronephrosis. In a prospective study, we found no clinically significant differences in sonograms performed in the first 48 hours of life and at 7 to 10 days of life (19). Therefore, in cases of bilateral hydronephrosis, suspected multicystic dysplastic kidney, or concern for loss to follow-up, an initial sonogram prior to discharge from the nursery appears reliable. In cases of suspected bladder outlet obstruction, it is mandatory to order the sonogram in the first day. Unilateral hydronephrosis can reliably be studied with an early sonogram, but the degree of hydronephrosis may change if the study is postponed a week. Follow-up studies are important.
in the first 48 hours of life because perinatal dehydration may prevent adequate distension of the urinary tract to detect hydronephrosis. In a prospective study, we found no clinically significant differences in sonograms performed in the first 48 hours of life and at 7 to 10 days of life (19). Therefore, in cases of bilateral hydronephrosis, suspected multicystic dysplastic kidney, or concern for loss to follow-up, an initial sonogram prior to discharge from the nursery appears reliable. In cases of suspected bladder outlet obstruction, it is mandatory to order the sonogram in the first day. Unilateral hydronephrosis can reliably be studied with an early sonogram, but the degree of hydronephrosis may change if the study is postponed a week. Follow-up studies are important.
Hydronephrosis is often graded using the system of the Society of Fetal Urology (Table 96-2) (23). In the absence of vesicoureteral reflux, if no hydronephrosis (grade 0) is seen on the initial postnatal sonogram, no further follow-up is necessary. Obstruction has not been found in cases of grade 1 to 2 hydronephrosis with long-term follow-up (24); however, these patients are typically followed with repeat sonograms every 6 to 9 months up to age 18 to 24 months to ensure no worsening of dilation occurs. In the higher grades of hydronephrosis (grades 3 to 4), whether detected at birth or later, obstruction must be excluded. Grade 4 hydronephrosis is particularly worrisome because thinning of the renal cortex is suggestive but not diagnostic for obstruction (Fig. 96-2). Nuclear renography has replaced intravenous pyelography to exclude obstruction because the former provides objective measures of differential renal function and drainage from the renal unit. Even with exclusion of obstruction, continued follow-up is mandatory because delayed obstruction can occur in the first 2 years of life (24). Routine sonography is performed in such cases every 3 months for the first year of life and every 6 months in the second year of life. The need for prolonged follow-up after 2 years in the absence of obstruction is not yet determined.
TABLE 96-2 Society of Fetal Urology Grading Scale for Hydronephrosis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
The search for a reliable test to diagnose obstruction of the upper urinary tract has included many different studies. High-grade hydronephrosis may exist in the absence of obstruction and only represent the sequela of obstruction that developed and resolved prior to birth. Currently, nuclear renography under standardized parameters represents the gold standard to diagnose obstruction. It is preferable to wait until the newborn is at least 1 month of age to perform the renogram. The glomerular filtration rate is believed to increase severalfold in the first few weeks of life, adding greater accuracy to the study (25), and a short delay in relief of obstruction, if present, is not believed to lead to irreversible damage (10). Technetium-99m-mercaptoacetyltriglycine (MAG-3) is the preferred radionuclide because, unlike technetium-99m-diethylenetriaminepentaacetic acid, it is both filtered and secreted by the nephron to provide superior imaging. One to 2 minutes following administration of the radiotracer, differential renal function is determined by measuring the radioactivity from each renal unit. Normal function from each side is considered 40% to 60% of total renal function.
The excretion of the radiotracer from the pelvis is then observed for 30 to 60 minutes and may assume one of several different patterns (Fig. 96-3).
The excretion of the radiotracer from the pelvis is then observed for 30 to 60 minutes and may assume one of several different patterns (Fig. 96-3).
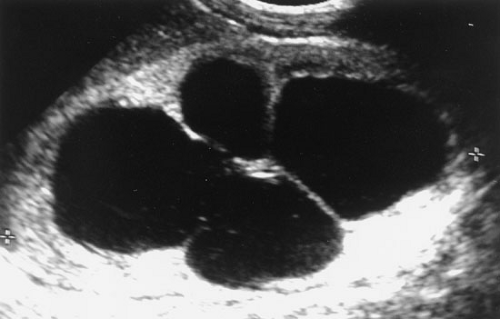 FIGURE 96-2. Postnatal sonogram of newborn with history of prenatal hydronephrosis. Grade 4 hydronephrosis with massive pelvocaliectasis with cortical thinning. |
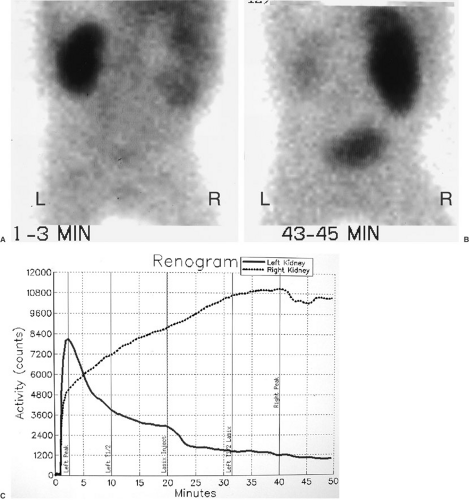 FIGURE 96-3. MAG-3 diuretic renogram in patient in Figure 96-2. (A) Initial images at 1 to 3 minutes show normal appearance of left kidney. The right kidney is enlarged with diffuse activity and photopenic renal hilum due to hydronephrosis. (B) Image at 43 to 45 minutes shows near complete drainage of left kidney and renal pelvis. The right kidney and renal pelvis show continued retention of tracer consistent with obstruction. (C) Washout curves show normal pattern for left kidney and unequivocally obstructed pattern of right kidney with no drainage. |
Postnatal Presentation
Older children with congenital or acquired obstructive uropathies can present in a variety of ways. A classical presentation of UPJ obstruction in adults is Dietl’s crisis—severe onset of flank pain, nausea, and vomiting, particularly after diuresis induced by excessive fluid intake, particularly alcohol or caffeine. This occurs because the rate of urine production exceeds the maximal possible flow rate across the point of critical narrowing, causing acute distension of the renal pelvis, which stretches and triggers impulses in the pain fibers of the renal capsule and autonomic splanchic nerves. Children certainly can present in this manner, but this is more likely with acquired ureteral narrowing, acute obstructing calculi, or extrinsic compression of the ureter. The chronic dilation associated with congenital obstruction may be less likely to produce symptoms. Some children with chronic obstruction will present with long-standing episodic bouts of abdominal pain and vomiting in the setting of a fruitless extensive gastrointestinal (GI) workup.
Gross hematuria after minor trauma can be seen in obstructive uropathy because vessels of the renal pelvis are dilated and more prone to bleeding. Microhematuria is found in most but not all patients with urolithiasis; gross hematuria is much less common.
Obstruction rarely presents with infection; however, UTIs are the second most common bacterial infections in children. Although there is no completely reliable method of differentiating bladder and kidney infections, fever, flank pain, nausea, vomiting, lethargy, and malaise would suggest the latter. Up to one-third to one-half of children presenting with febrile UTIs will have some abnormality of their urinary tract (26). For this reason, it is generally recommended to perform a VCUG and renal/bladder sonogram on children following a febrile UTI. The utility of these studies in older children and the timing of this study following infection is controversial (26,27). Vesicoureteral reflux is by far the most common abnormality detected in workups following febrile UTIs. Obstruction of the upper urinary tract presents with infection much less commonly because the bacteria must not only ascend the urethra to cause a bladder infection, but must also ascend the ureter past the point of obstruction to gain access to the upper urinary tract. Infection in the face of obstruction can be more serious with possible development of pyonephrosis or abscess due to bacterial growth in an essentially closed space. Sonography may be required to rule out such pathology if a febrile UTI does not rapidly respond to antibiotic therapy.
SPECIFIC DIAGNOSES OF OBSTRUCTIVE UROPATHY
Ureteropelvic Junction Obstruction
UPJ obstruction is the most common form of obstructive uropathy and should be corrected when present to prevent progressive renal insult. The decision to operate is fairly straightforward in those patients who present with symptoms, particularly recurrent flank pain associated with nausea and vomiting. Pyelonephritis on the affected side is also a relatively compelling indication for correction. Unfortunately, for the clinician, symptomatic presentation in children is rare. In the era of prenatal sonography, the vast majority of cases of UPJ obstruction in children present without symptoms but because of prenatal hydronephrosis (28). Much debate has centered over the presence of hydronephrosis and the relative indication for correction of UPJ obstruction because there is no definitive definition of UPJ obstruction.
There appears to be a slight preponderance of UPJ obstruction in males, representing approximately two-thirds of cases. Obstruction on the left is more common with a ratio of 3:2 versus the right side. Bilateral UPJ obstruction may exist in 5% to 15% of cases. If obstruction is found in a kidney with duplication of the collecting system, it almost always exists in the lower pole moiety (10,28).
The causes of UPJ obstruction are classified as intrinsic, extrinsic, and secondary. Intrinsic lesions of the ureter and its junction with the renal pelvis cause obstruction by constricting the lumen. Interruption in the development of the circular musculature and an excessive deposition of collagen fibers between muscle cells at the UPJ have been demonstrated to cause a thickening of the ureteral wall (28). Rare causes of intrinsic UPJ obstruction include congenital valvular mucosal folds, persistent fetal convolutions, and upper ureteral polyps. Extrinsic UPJ obstruction is most commonly caused by an aberrant additional vessel to the lower pole of the kidney kinking the UPJ or upper ureter as it passes to (or from) the great vessels. Because moving the ureter from the course of the crossing vessel does not always relieve the obstruction, it is not clear whether the vessel coexists with a preexisting intrinsic lesion or is the cause of the intrinsic lesion. The presentation of UPJ obstruction due to an obstructing crossing vessel is more common in older children and with symptoms of pain and vomiting (29). Intrinsic obstruction due to abnormal fetal ureteral development is seen more commonly in neonates and younger children, whereas an aberrant crossing vessel may not cause obstruction until the child has grown and the spatial relationship between ureter and vessel changes and causes kinking. Differentiation between intrinsic and extrinsic lesions may be difficult,
and the diagnosis is usually not established until exploration at the time of repair.
and the diagnosis is usually not established until exploration at the time of repair.
The advent of prenatal sonography led to a dramatic increase in the number of infants presenting with hydronephrosis and possible UPJ obstruction. Pyeloplasties, therefore, were performed on many of these newborns to correct the perceived obstruction (30). It was noted, however, that renal function was not altered in most of these patients at the time of workup (31). If renal function was found to be diminished, it may have been irreversibly altered prior to birth, and postnatal surgery did not improve function (32). This led some investigators to follow such newborns expectantly, unless renal function was initially diminished or diminished during observation (31,33). Only 22% to 25% of observed newborns with severe unilateral hydronephrosis required subsequent pyeloplasty; therefore, nearly three-fourths of these neonates with apparent UPJ obstruction on ultrasound could be followed safely without obvious deleterious effect. Of those not undergoing surgery, 69% had resolution of the hydronephrosis and 31% had improvement. This suggests that most newborns with severe unilateral hydronephrosis can be followed; obstruction demonstrated by loss of renal function on nuclear renal scan is found in only a minority. In most, the hydronephrosis detected is a sequela of fetal maldevelopment and will resolve over time. In a minority, function will deteriorate, perhaps as the critical degree of obstruction cannot accommodate the urine flow of the growing and developing infant kidney. Such obstruction is typically silent; therefore, these patients must be followed rigorously to determine which patients may or may not need surgical correction. The usual indications for surgery in follow-up are worsening hydronephrosis or worsening function on nuclear renal scan (33).
Once the decision to surgically correct UPJ obstruction is made, open pyeloplasty is the conventional procedure. This procedure has been shown to be highly successful with success rates of greater than 95%, even in newborns (10). The renal pelvis and ureter can be exposed extraperitoneally through a muscle-dividing flank incision made anteriorly from the tip of the twelfth rib or a muscle-splitting dorsal lumbotomy incision through the back between the twelfth rib and bony pelvis. The dorsal lumbotomy may be less morbid to the patient, but can limit the surgeons’ exposure (34,35). The renal pelvis and upper ureter should be exposed by dissecting in a plane just superficial to its adventitia to preserve the intrinsic blood supply. The repair is usually a dismembered (Anderson-Hynes) pyeloplasty; this involves division of the UPJ, resection of the stenotic segment and/or rerouting the ureter around an aberrant crossing vessel, wide spatulation of the proximal ureter, and careful anastomosis of the pelvis to the ureteral segment with fine absorbable suture (Fig. 96-4). If a large redundant pelvis is present, the pelvis is resected generously to prevent kinking at the ureteropelvic anastomosis when filling occurs. If the length of the obstructing segment is more than 1.5 to 2 cm, a nondismembered pyeloplasty using a flap of renal pelvic tissue to augment the narrowed segment can be employed. Ureterocalycostomy is usually reserved for cases in which dependent renal drainage across the UPJ cannot be established by the conventional techniques (e.g., in the setting of fused kidneys or previous surgery). In this procedure, amputation of the lower pole cortex is performed, and the most dependent calyx is sewn to the ureter in a tension-free mucosa-to-mucosa anastomosis (36).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree