Pediatric Renal Transplantation
Karen M. Kling
Department of Pediatric Surgery, Johns Hopkins University School of Medicine, Johns Hopkins Hospital, Baltimore, Maryland.
Pediatric renal transplants are performed 5 to 600 times annually in the United States (1). When compared with dialysis, transplantation improves survival, growth, sexual development, and cognitive function. Advances in surgical technique, postoperative management, and immunosuppression have led to excellent outcomes in even very small children with overall patient and graft survival up to 97% and 92%, respectively (2).
ETIOLOGY OF RENAL FAILURE
Forty-five percent of pediatric end-stage renal disease (pESRD) is congenital and includes obstructive urological and dysplastic renal conditions (Table 47-1). Focal segmental glomerular sclerosis (FSGS) is the third leading cause of pESRD overall, but it is the most common cause in adolescent African Americans. Other causes include reflux-related infection, prune belly, nephrotic syndrome, hemolytic uremic syndrome, cystinosis, oxalosis, glomerulonephritis, lupus nephritis, renal infarction, Wilms’ tumor, diabetic nephropathy, and medication toxicities. The etiology of pESRD is uncertain in about 5% of cases. Caucasians represent 60% of recipients with the rest divided evenly between African American and Hispanic patients. Males more frequently have obstructive uropathies and account for 60% of recipients (1). Most patients are school age or adolescent and children younger than 6 years of age comprise only 20% of transplant recipients.
INDICATIONS FOR TRANSPLANTATION
A glomerular filtration rate less than 10 mL per minute per 1.73 m2 mandates renal replacement therapy via dialysis or transplantation. Children may require transplantation before this if they have severe growth failure, uncontrollable hyperparathyroidism, bone loss, refractory anemia, or dialysis access limitations. Many wait for patients to exceed 10 kg or 24 months of age before transplantation because historically children younger than 2 years had outcomes inferior by up to 20% compared with overall results (3,4); more recent data suggest that this may be improving. Preemptive transplantation is done in 24% (1) of cases, and outcomes are slightly improved compared with cadaveric data. This also abrogates the burden, risk, and cost of dialysis. Active malignancy and HIV have been considered contraindications to transplantation due to potential risks of immunosuppression. In patients with complex medical conditions, quality of life should be considered in determining whether transplantation will be of benefit.
PREOPERATIVE PREPARATION
Patients must be medically optimized with respect to hypertension, volume, anemia, nutrition, calcium/phosphate homeostasis, and immunization. They must be psychologically ready to manage drug regimens and potential complications. Satisfactory urologic function must be present and because children with previous transplants are more common, one must determine the location of previous transplants and whether the vasculature remains adequate.
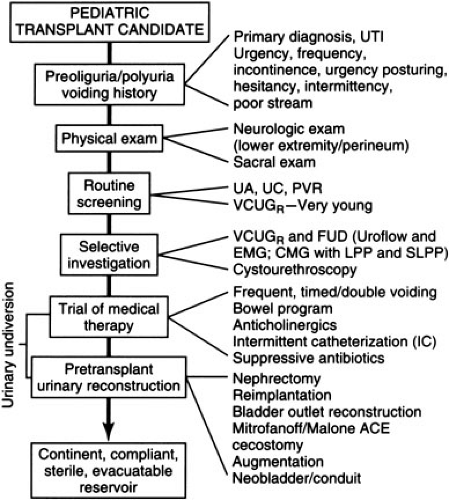
Urologic reconstruction is indicated to ensure continence, compliance, sterility, and functional evacuation of the urinary tract (5). It is essential to ascertain the nature of previous urological reconstruction to anticipate problems with exposure or blood supply (Fig. 47-1). History of urinary infections, voiding symptoms, hydronephrosis, abnormal bladder wall thickness, or an abnormal sacral neurological exam increases the suspicion for potential urologic problems and mandates workup to rule out inadequate bladder capacity, poor voiding, reflux, or obstruction. This is accomplished via voiding cystourethrogram and measurement of post void residual; patients may also require urodynamic studies or cystourethroscopy. In
patients with a history of posterior urethral valves, it is important to document relief of their urethral obstruction. If bladder volume is less than 100 cc or voiding pressure is greater than 100 cm H2O, reconstruction should be considered because high intravesicular pressure has been associated with graft dysfunction; congenitally small bladders may require augmentation via ileocystoplasty, colocystoplasty, gastrocystoplasty, or intestinal conduit. Small but distensible bladders of oliguric patients with normal anatomy readily accommodate the increased volume of urine after transplantation. Reflux should be corrected and those with poor emptying, especially in the face of reflux, must have a dependable method for bladder emptying, which may involve self-catheterization or creation of a continent stoma. Major reconstruction should be performed prior to transplant before the administration of systemic steroids and so mechanical problems do not contribute to postoperative graft dysfunction. Extraperitoneal approaches are preferable to preserve the peritoneal membrane for dialysis. Posttransplant reconstruction should be performed after graft function normalizes and prednisone is tapered to 0.1 to 0.2 mg per kg per day.
patients with a history of posterior urethral valves, it is important to document relief of their urethral obstruction. If bladder volume is less than 100 cc or voiding pressure is greater than 100 cm H2O, reconstruction should be considered because high intravesicular pressure has been associated with graft dysfunction; congenitally small bladders may require augmentation via ileocystoplasty, colocystoplasty, gastrocystoplasty, or intestinal conduit. Small but distensible bladders of oliguric patients with normal anatomy readily accommodate the increased volume of urine after transplantation. Reflux should be corrected and those with poor emptying, especially in the face of reflux, must have a dependable method for bladder emptying, which may involve self-catheterization or creation of a continent stoma. Major reconstruction should be performed prior to transplant before the administration of systemic steroids and so mechanical problems do not contribute to postoperative graft dysfunction. Extraperitoneal approaches are preferable to preserve the peritoneal membrane for dialysis. Posttransplant reconstruction should be performed after graft function normalizes and prednisone is tapered to 0.1 to 0.2 mg per kg per day.
TABLE 47-1 Etiology of ESRD in Pediatric Transplant Recipients. | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
Preservation of urine output maintains bladder mechanics and may decrease dialysis dependence. Native kidneys should not be removed except for the following indications: chronic infection, refractory hypoalbuminemia or hypercoaguability of nephrotic syndrome (6), refractory hypertension, tumor (bilateral Wilms’), mass effect (polycystic kidneys), and hypovolemic polyuria. Nephrectomy should be performed 1 to 2 months before transplantation to improve nutrition, coagulation, or infectious status. Nephrectomy (hypertension) can be performed simultaneously with transplantation, but operative time is prolonged and retroperitoneal dissection occurs just prior to systemic heparinization; in addition, decreases in blood pressure and volume shifts may compromise blood flow to the graft. A flank incision with retroperitoneal approach allows the best exposure to afford complete nephroureterectomy as is necessary in patients with reflux. A posterior approach is useful for bilateral nephrectomies of small kidneys, avoids the peritoneal cavity, and has very little postoperative discomfort. The transverse upper abdominal incision for bilateral nephrectomy should be avoided because it violates the peritoneal cavity and may compromise the blood supply when a second transverse retroperitoneal incision is used for the transplant. Transabdominal resection may be useful if one is intraperitoneal for another reason, but transabdominal laparoscopic or open approaches are of limited use in patients dependent on peritoneal dialysis. Removal of a failed graft is indicated for the previous reasons or if there is significant graft pain, hematuria, acute rejection, and complications of immunosuppression. Some believe that high levels of antibodies due to persistent exposure to graft antigens warrant graft removal in preparation for future transplantation.
Parathyroidectomy may be required in the setting of tertiary hyperparathyroidism to curb renal osteodystrophy. Surgical or endoscopic gastrostomy may be necessary for pretransplant volume replacement or caloric supplementation. Vaccinations including hepatitis B should be given prior to transplantation; live virus vaccines are not advised
in the immunosuppressed. Education is paramount to successful transplantation to prepare patients for the social, financial, and psychological ramifications of their new chronic condition and to reinforce the importance of compliance with medicinal, dietary, and lifestyle regimens. There is a well-documented increase in noncompliance in adolescent patients with increased graft loss as a consequence.
in the immunosuppressed. Education is paramount to successful transplantation to prepare patients for the social, financial, and psychological ramifications of their new chronic condition and to reinforce the importance of compliance with medicinal, dietary, and lifestyle regimens. There is a well-documented increase in noncompliance in adolescent patients with increased graft loss as a consequence.
The most important factors for graft suitability have been blood group antigen (ABO) and human leukocyte antigen (HLA) matching. Antibodies against mismatched proteins lead to hyperacute rejection and immediate irrecoverable graft thrombosis. The greater the HLA match, the better the outcome; however, with potent immunosuppression and living related transplantation, lesser degrees of HLA matching have demonstrated good outcomes. Living donor (LD) grafts have outcomes several percentage points above cadaveric (CAD) grafts. Graft selection should take into account the quality of the donor organ, HLA match, and likelihood that there will be access to other timely and suitable grafts. Donor issues that negatively affect graft function include history of hypertension or diabetes, cerebrovascular accident, elevated creatinine, greater than 20% glomerulosclerosis, prolonged ischemia time, and age younger than 6 years or older than 55 years. Cold ischemia time has one of the strongest influences on function, and ischemia greater than 24 hours is associated with significant acute tubular necrosis (ATN) and delayed graft function (DGF), which is associated with decreased graft longevity (1). If longer ischemia times are anticipated, ex vivo perfusion has been used to improve preservation, decrease DGF, and provide flow measurements that may predict graft function.
Living donor transplantation is increasing and currently accounts for 51% of pediatric transplants (1). This allows for medical optimization of the donor and recipient, earlier transplant, control of perioperative volume status, and negligible cold ischemia time. Decreased rejection is seen with LD grafts, and data demonstrate a 6% and 10% greater graft survival at 1 and 3 years, respectively, for LD versus CAD patients (1). Living donation increases the graft pool but subjects a healthy person to an operation. Therefore, donor evaluation needs assure both good graft function and fitness of the donor to undergo nephrectomy with adequate residual renal function. The donor must be free of hepatitis, tuberculosis, and HIV. Serious morbidity in donors occurs in less than a few percent and mortality is much less than 1%. Laparoscopic donor nephrectomy affords decreased length of stay, faster return to work, and decreased pain (7).
OPERATIVE TECHNIQUE
After the patient is anesthetized, access for central venous pressure (CVP) monitoring is placed; invasive arterial monitoring is not essential in pediatric patients unless they have poorly regulated hypertension, very small size, anticipated aortic cross-clamping, or other medical conditions that warrant monitoring. We avoid epidural anesthetic infusion for infants as the sympathectomy may decrease perfusion to the graft. A sterile urinary catheter is inserted, and then the bladder is irrigated and distended with 5 to 10 cc per kg of saline. Peritoneal dialysis catheters should be drained to empty the peritoneal compartment. A roll may be placed under the hip on the side of implantation to elevate the pelvis and vessels. An intravenous cephalosporin should be given and intravenous steroid (10 mg per kg) and any other induction agents should be administered at this time. Heparin, mannitol (0.25 mg per kg), furosemide (1 to 2 mg per kg), dopamine, and volume expanders should also be available. Blood transfusion is rarely necessary.
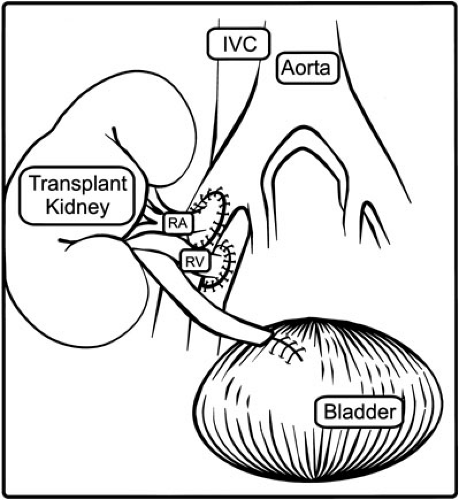
A retroperitoneal approach to the right or left flank is appropriate; a right-sided approach provides access to the distal aorta and vena cava for implantation in a small child. Generous mobilization of the peritoneum medially to expose the great vessels will create enough space to admit an adult kidney even in an infant. A transabdominal approach in unnecessary even in small patients, but may be useful if multiple prior transplants occupy both iliac fossae. Previous allografts, catheter exit sites, or urologic operations may favor one side over the other; however, matching the side of the graft to the side of the recipient bed is an unnecessary restriction. A curvilinear lateral abdominal incision is carried down through the abdominal wall, and the retroperitoneal space is entered. The peritoneum and its contents are swept medially. The retroperitoneal space need only be developed enough to accommodate the kidney, prepare the vessels, and expose the anterolateral surface of the bladder. With a stationary retractor in place, the iliac, aortic, or caval vessels are isolated for anastomosis and placement of clamps. Substantial lymphatics should be ligated to avoid postoperative lymphocele.
The graft, previously flushed with iced preservation solution, is kept on ice while its vessels are prepared for anastomosis and excess perinephric fat is removed; periureteral tissue is left intact to avoid devascularization. The recipient is then given 75 to 100 units per kg of heparin and the recipient vessels are clamped. Infants weighing less than 15 kg usually require anastomosis to the distal aorta and vena cava, with the caval anastomosis at the junction between the cava and the right iliac vein. Young children usually have sufficient common iliac vessels; in older children and adolescents, the external iliac vessels may be appropriate. The anastomoses are performed in an end donor to side recipient fashion (Fig. 47-2) using running monofilament suture. Multiple arteries of substantial size (greater than 2 mm) should be reconstructed ex vivo to form a common orifice. Arteries that cannot be joined may be implanted independently. Vessels of insignificant
caliber or distribution can be ligated. When the anastomoses are nearly complete, furosemide and mannitol are given to promote diuresis through the kidney. One should verify that reperfusion to the graft will occur under optimal conditions, and this requires a CVP of 13 to 18 cm H2O and a systolic blood pressure of 120 to 130 mm Hg. One should anticipate that 250 cc of circulating volume (up to one-third of a 10-kg child’s blood volume) will be sequestered in the graft on reperfusion with a concomitant drop of 4 to 5 cm H2O in CVP. Even with manipulation of volume and blood pressure, small pediatric recipients of adult grafts demonstrate decreased transplant renal artery blood flow (8). Some surgeons inject a calcium channel blocker into the proximal artery or Papaverin into the adventitia to abrogate any vasospasm. The best assessment of graft perfusion is palpation; a graft that immediately becomes turgid, pulsatile, and pink is well perfused, but if the graft becomes flaccid or loses pulse one must verify good inflow. Embolectomy or anastomotic revision may be required if thrombus or dissection are evident. To increase perfusion to the graft, volume expansion and dopamine may be helpful. A well-perfused graft with a reasonable ischemia time should readily make urine. If perfusion is intact but there is no urine output, reversible ATN is the most likely diagnosis. One should verify a cross-match as antibody-mediated rejection treatable with plasmapheresis. At this juncture, hemostasis should be ascertained by examining the anastomoses, capsule, and hilar fat.
caliber or distribution can be ligated. When the anastomoses are nearly complete, furosemide and mannitol are given to promote diuresis through the kidney. One should verify that reperfusion to the graft will occur under optimal conditions, and this requires a CVP of 13 to 18 cm H2O and a systolic blood pressure of 120 to 130 mm Hg. One should anticipate that 250 cc of circulating volume (up to one-third of a 10-kg child’s blood volume) will be sequestered in the graft on reperfusion with a concomitant drop of 4 to 5 cm H2O in CVP. Even with manipulation of volume and blood pressure, small pediatric recipients of adult grafts demonstrate decreased transplant renal artery blood flow (8). Some surgeons inject a calcium channel blocker into the proximal artery or Papaverin into the adventitia to abrogate any vasospasm. The best assessment of graft perfusion is palpation; a graft that immediately becomes turgid, pulsatile, and pink is well perfused, but if the graft becomes flaccid or loses pulse one must verify good inflow. Embolectomy or anastomotic revision may be required if thrombus or dissection are evident. To increase perfusion to the graft, volume expansion and dopamine may be helpful. A well-perfused graft with a reasonable ischemia time should readily make urine. If perfusion is intact but there is no urine output, reversible ATN is the most likely diagnosis. One should verify a cross-match as antibody-mediated rejection treatable with plasmapheresis. At this juncture, hemostasis should be ascertained by examining the anastomoses, capsule, and hilar fat.
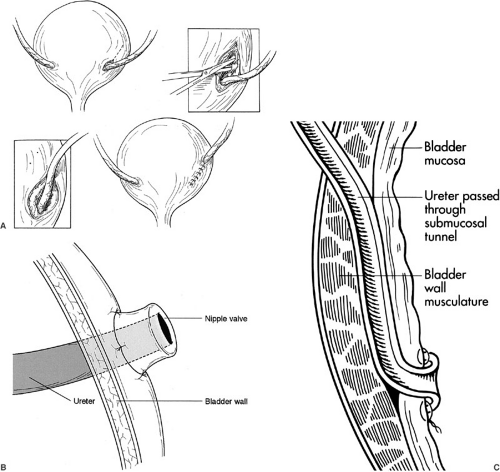
The distended bladder should be easily identified by palpating the catheter balloon inside. The transplant ureter should be trimmed of devascularized tissue and tunneled under the cord structures without twisting. An appropriate position on the anterolateral aspect of the bladder is selected given that when emptied it will withdraw into the pelvis. A variety of methods for neoureterocystotomy can be used. The Lich (Gregoir-Liche) extravesicle approach involves dividing the muscular layer of the bladder to expose the bulging mucosa (Fig. 47-3A). After opening the mucosa, an anastomosis is created between the ureteral and bladder mucosa. A muscular tunnel is closed over the mucosal anastomosis to prevent reflux; a 4:1 ratio for tunnel length to ureteral diameter is recommended. A detrusorrhaphy can also be performed in which the previous technique is varied so the distal tip of the ureter is advanced into the bladder with an apical stitch to leave a nonrefluxing ureteral orifice floating within the bladder. The muscular layer is closed over the ureters, occasionally incorporating a small bite of ureteral wall to buttress the apical ureteral stitch. Alternatively, a nipple can be created by turning the end of the ureter out on itself, placing the end of the nipple into the bladder, and fashioning the turned back mucosal edge of the ureter to the recipient bladder mucosa with a running mucosal suture line (Fig. 47-3B). The Leadbetter-Politano technique involves creation of an anterior cystotomy through which a submucosal tunnel of about 2.5 cm is created (Fig. 47-3C). The donor ureter is brought through the outer muscular layer and under the mucosal tunnel, and then a mucosal anastomosis is fashioned from the inside of the bladder from donor ureter to bladder mucosa near the trigone. Regardless of the technique, it is essential to approximate the mucosa using absorbable suture placed closely together because as the bladder distends the suture line will stretch. Also, the mucosal tunnel must not obstruct urine flow or compromise ureteral blood supply because this will lead to devascularization and complete disruption of the anastomosis several days after surgery. Less obstruction (1% vs. 4%) but more leakage and reflux occur with the Lich repair when compared with the Leadbetter-Politano; the nipple valve technique also has low rates of reflux, but a higher propensity for obstruction. If the bladder is not accessible, ureteroureteral anastomosis can also be performed as long as the recipient still has a patent nonrefluxing ureter. Ureteral stents are unnecessary unless there is concern about devascularization of the ureter (i.e., transected lower pole artery), abnormal bladder tissue, or previous radiation.
The graft is oriented to prevent compromised blood flow and to facilitate future percutaneous biopsy. Drains are
unnecessary unless there is significant lymphatic drainage or coagulopathy. The abdominal wall is closed, taking care not to injure the peritoneum; in very small children, only the external layer is closed to afford sufficient but relaxed closure. Before leaving the operating room one should confirm that urine output is not diminished and that ipsilateral pedal pulses are intact. Peritoneal dialysis catheters must be removed before discharge as they pose a risk for infection, but we have chosen to remove them in a separate procedure after sufficient graft function is assured. Maintaining peritoneal dialysis access is important to consider, especially in children weighing less than 10 kg because they may need considerable volume expansion to support initial graft function, they are unable to tolerate significant volume overload, their grafts may not function immediately, and hemodialysis can be treacherous for children of this size. In a larger child whose graft has excellent prognosis, who makes urine immediately, and who could tolerate hemodialysis, it is acceptable to remove the catheter at the time of transplantation.
unnecessary unless there is significant lymphatic drainage or coagulopathy. The abdominal wall is closed, taking care not to injure the peritoneum; in very small children, only the external layer is closed to afford sufficient but relaxed closure. Before leaving the operating room one should confirm that urine output is not diminished and that ipsilateral pedal pulses are intact. Peritoneal dialysis catheters must be removed before discharge as they pose a risk for infection, but we have chosen to remove them in a separate procedure after sufficient graft function is assured. Maintaining peritoneal dialysis access is important to consider, especially in children weighing less than 10 kg because they may need considerable volume expansion to support initial graft function, they are unable to tolerate significant volume overload, their grafts may not function immediately, and hemodialysis can be treacherous for children of this size. In a larger child whose graft has excellent prognosis, who makes urine immediately, and who could tolerate hemodialysis, it is acceptable to remove the catheter at the time of transplantation.
POSTOPERATIVE MANAGEMENT
Renal transplant patients require meticulous attention to volume status, blood pressure, electrolytes, and urine
output, and must be in an environment in which appropriate CVP monitoring and nursing resources exist. Successful transplantation of adult grafts into infants requires generation of high aortic blood flow by expanding circulating volume and increasing systolic pressure sometimes with dopamine; significant volume administration may postpone extubation. Even doubling infant aortic blood flow, transplanted renal artery flows are only two-thirds of their pretransplant donor flow (8) and the effects on recovery from ATN and long-term function are not well established. It is important to maintain systolic blood pressures between 120 and 130 mm Hg and CVP of 10 to 15 mm H2O. Maintaining adequate volume can be challenging because patients may produce several liters of urine daily due to the transplanted kidney’s poor concentrating ability and due to native kidney output. Volume management is best accomplished by providing fluid for insensible losses and replacing hourly urine output with isotonic saline, adjusting the composition of fluid based on serum and urine electrolytes. Patients whose urine output does not respond may require colloid administration. As CVP increases, one must wean replacements judiciously before volume overload makes diuresis necessary. One must also be careful to avoid zealous volume expansion in the face of an oliguric graft, which may then precipitate the need for dialysis that in turn causes fluid shifts undesirable in the peritransplant period. In those who do not make urine despite adequate systemic hemodynamics, one must immediately rule out vascular or urologic problems (Fig. 47-4A). In patients who made urine preoperatively, urine output can be deceiving because this may be due to the native kidneys’ response to intraoperative volume and diuretics. If serum creatinine does not fall by about one-half in 36 hours, one must rule out technical problems. Electrolytes, serum urea nitrogen, and creatinine levels are monitored every 6 hours for the first 24 hours, then daily following that. Duplex ultrasound of the transplant vessels is performed routinely on the first postoperative day (unless a preoperatively anuric patient is making copious urine) or any time the vasculature is of concern.
output, and must be in an environment in which appropriate CVP monitoring and nursing resources exist. Successful transplantation of adult grafts into infants requires generation of high aortic blood flow by expanding circulating volume and increasing systolic pressure sometimes with dopamine; significant volume administration may postpone extubation. Even doubling infant aortic blood flow, transplanted renal artery flows are only two-thirds of their pretransplant donor flow (8) and the effects on recovery from ATN and long-term function are not well established. It is important to maintain systolic blood pressures between 120 and 130 mm Hg and CVP of 10 to 15 mm H2O. Maintaining adequate volume can be challenging because patients may produce several liters of urine daily due to the transplanted kidney’s poor concentrating ability and due to native kidney output. Volume management is best accomplished by providing fluid for insensible losses and replacing hourly urine output with isotonic saline, adjusting the composition of fluid based on serum and urine electrolytes. Patients whose urine output does not respond may require colloid administration. As CVP increases, one must wean replacements judiciously before volume overload makes diuresis necessary. One must also be careful to avoid zealous volume expansion in the face of an oliguric graft, which may then precipitate the need for dialysis that in turn causes fluid shifts undesirable in the peritransplant period. In those who do not make urine despite adequate systemic hemodynamics, one must immediately rule out vascular or urologic problems (Fig. 47-4A). In patients who made urine preoperatively, urine output can be deceiving because this may be due to the native kidneys’ response to intraoperative volume and diuretics. If serum creatinine does not fall by about one-half in 36 hours, one must rule out technical problems. Electrolytes, serum urea nitrogen, and creatinine levels are monitored every 6 hours for the first 24 hours, then daily following that. Duplex ultrasound of the transplant vessels is performed routinely on the first postoperative day (unless a preoperatively anuric patient is making copious urine) or any time the vasculature is of concern.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree