Liver, Intestine, and Multivisceral Transplantation in Children
Jorge Reyes
Transplant Division, University of Washington Medical Center, Seattle, Washington 98195.
Clinical solid organ transplantation in children has spanned more than 40 years, increasing exponentially since the mid-1980s. Children account for 381 cadaveric kidney transplants (4.4% of 8,662), 478 liver transplants (8.9%), and 287 heart transplants (14%) performed in the United States in the year 2003. However, liver disease continues to be the leading indication for organ transplantation in children, representing 37% of all recipients in 2003 (1).
Patient and graft survival rates have improved dramatically, principally due to advances in surgical expertise, perioperative care, and immunosuppressive management. Current United Network for Organ Sharing 1-year patient survival rates for children are 91.2% to 94.6% for cadaveric kidney transplants, 75.1% to 84.5% for liver transplant recipients, and 83.6% to 92.8% for heart transplant recipients. Pediatric patients comprise 0% to 1.2% of the kidney, pancreas, and kidney pancreas waiting list; 4.6% to 6.4% of the liver, heart, and lung waiting list; and 15.4% of the heart/lung waiting list in the year 2003 (1,2).
This review examines the basis for successful clinical liver and intestinal transplantation, and includes updates on current indications, timing of transplant, technical innovations, and immunosuppressive management after abdominal organ transplantation.
LIVER TRANSPLANTATION
Orthotopic liver transplantation is the established therapy for the management of children with life-threatening irreversible liver disease. With current survival rates greater than 90% at 1 year at the more experienced centers, the indications and timing for transplantation have become broader. This has been a result of improvements in the transplant procedure such as reduced organ, split grafts, and living related donors, and also to improvements in immunosuppressive management and surveillance of infections disease complications.
INDICATIONS
The disease-specific indications for liver transplantation in children are as follows:
Metabolic diseases: alpha1-antitrypsin deficiency; tyrosinemia; glycogen storage disease type 4, 3, 1; Wilson’s disease; perinatal hemochromatosis
Acute and chronic hepatitis, fulminant hepatic failure, viral, toxin- or drug-reduced
Chronic active hepatitis with cirrhosis: hepatitis B, hepatitis C, autoimmune idiopathic
Intrahepatic cholestasis: idiopathic neonatal hepatitis, Alagille’s syndrome (bile duct paucity syndrome), familial intrahepatic cholestasis (Byler’s disease)
Obstructive biliary tract disease: extrahepatic biliary atresia, sclerosing cholangitis, traumatic and postsurgical biliary tract disease
Miscellaneous: cryptogenic cirrhosis, congenital hepatic fibrosis, Caroli’s disease, cystic fibrosis, cirrhosis secondary to prolonged total parenteral nutrition (TPN)
Extrahepatic biliary atresia is the most common indication (50%), followed by metabolic inborn errors of metabolism, acute hepatic necrosis, and autoimmune and cholestatic disorders. Children with biliary atresia who do not achieve successful biliary drainage will require liver transplantation within the first year of life. Some children with early successful drainage may require transplantation later due to uncontrollable ascites or portal hypertensive variceal bleeding. The inborn errors of metabolism may cause structural damage to the liver, but some diseases
principally manifest their hepatic enzyme deficiency with devastating effects on the brain or kidneys (Table 48-1). Acute hepatic necrosis comprises approximately 10% of cases. Often a precise etiology cannot be found; however, viruses, drugs, toxins, and metabolic diseases may be involved. Primary hepatic malignancies comprise a small but important portion of patients requiring liver transplantation because, in such patients with hepatocellular carcinoma or hepatoblastoma confirmed to the liver, transplantation provides the only hope for cure.
principally manifest their hepatic enzyme deficiency with devastating effects on the brain or kidneys (Table 48-1). Acute hepatic necrosis comprises approximately 10% of cases. Often a precise etiology cannot be found; however, viruses, drugs, toxins, and metabolic diseases may be involved. Primary hepatic malignancies comprise a small but important portion of patients requiring liver transplantation because, in such patients with hepatocellular carcinoma or hepatoblastoma confirmed to the liver, transplantation provides the only hope for cure.
TABLE 48-1 Metabolic Diseases for Which Liver Transplantation May Be Indicated. | |
|---|---|
|
Despite a clear consensus on the disease states that require liver transplantation, the need and timing for transplant has depended in large part on manifestations of end-organ failure such as portal hypertension, coagulopathy, and infection. Improving results with liver transplantation for children, however, has expanded the symptom/complex indicators for liver transplantation in children and are as noted in Table 48-2. Because children are expected to have a longer life span into adulthood and old age, it is important to achieve successful organ replacement before significant quality of life indicators, such as growth and development, are compromised.
Contraindications to liver transplantation have been limited, given the improved survival rates with this procedure in children, and may include uncontrolled infection of extrahepatic origin (principally line sepsis or pneumonia), severely disabling disease of the heart, lungs, or central nervous system, and extrahepatic and uncontrolled malignancy. There are currently no limitations on age or weight for transplantation because results after transplantation for children younger than 1 year and weighing less than 10 kg have considerably improved, thereby making this less of a risk factor. This has been a consequence of improvements in surgical technique, which include microvascular surgery and the ability to use segmental liver allografts as with split liver, reduced size, or living related donor procedures.
TABLE 48-2 Symptom/Complex Indications for Liver: Transplantation in Children. | |
|---|---|
|
Technical Advances from the Donor Procedure
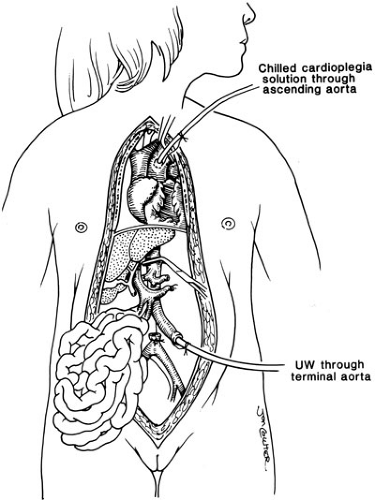
Successful engraftment of all solid organ allografts relies on appropriate preservation and procurement techniques, and allows for removal of all thoracic and abdominal organs including the liver. This is based on limited dissection of vascular inflow and outflow structures in situ, and then core cooling by rapid infusion of a chilled preservation solution into the donor aorta (Fig. 48-1) (3).
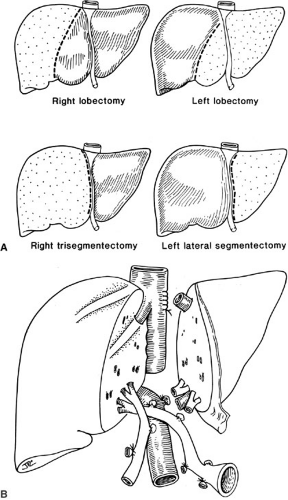
Special preservation solutions have been evolving since 1976; presently, the University of Wisconsin (UW) or Belzer solution is used routinely for all abdominal organ retrievals, providing preservation of the liver for as long as 24 hours (4). The need for reduction hepatectomy where a larger adult size liver allograft is reduced to fit a child may be necessary because finding appropriately size-matched donors for children is difficult; this has dramatically decreased the mortality in children with liver disease who are waiting for a liver. Reductions of the liver can be performed ex vivo as a back table procedure. The liver segment removed (right lobe or left lateral segment) will depend on the size mismatch between donor and recipient. The mere reduction of a liver graft, however, will remove this allograft from the donor pool to be used in an adult. Consequently, to address the donor organ scarcity, the next innovation was inevitably the splitting of a liver graft to transplant two patients, and also living related liver transplantation (Fig. 48-2) (5).
Split liver transplantation in which one donor liver allograft is transplanted into two recipients (usually an adult and a child) can be performed either ex vivo as a back table procedure after procurement of the whole liver or in situ prior to perfusion in the hemodynamically stable cadaveric donor (Fig. 48-2). With this procedure, 1-year pediatric patient survival compares favorably with whole organ transplantation (6,7).
Living related donation uses similar liver reduction strategies; with pediatric liver transplantation, generally the left lateral segment or left lobe is removed from an adult patient. However, occasionally for larger children and adolescents, the right lobe may also be used. These segments are isolated in the donor with minimal manipulation of the graft and perfused immediately as a back table procedure (8).
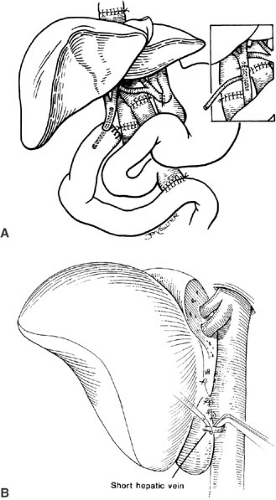
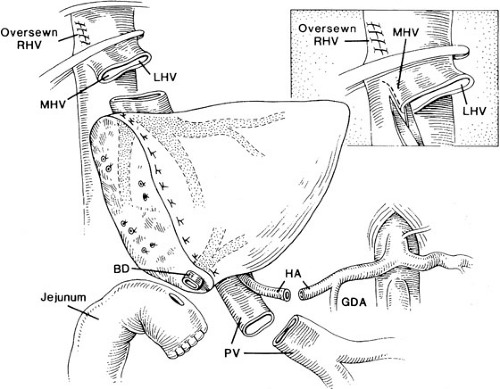
The implantation of recipient operation segmental grafts is similar to whole organ transplantation and generally involves using a “piggyback” technique (Fig. 48-3), which preserves the retrohepatic vena cava. The hepatic vein outflow of the segmental graft is attached to the common conduit of hepatic veins, and the graft hepatic artery and portal vein are anastomosed to the corresponding recipient vessels. Generally, a Roux-en-Y limb is used to reconstruct the biliary drainage (Fig. 48-4).
The standard liver transplant encompasses four anastomosis: (1) of the suprahepatic vena cava, (2) the portal vein, (3) the hepatic artery, and (4) the biliary reconstruction. Modifications of the procedure generally involve the use of vascular homografts from a cadaveric donor to the infrarenal aorta of the recipient for a neohepatic artery, and to the superior mesenteric vein or confluence of splenic vein and superior mesenteric vein to serve as a new portal vein. Such variations in arterial and portal vein reconstruction are dependent on donor/recipient anomalies and on the occurrence of vasculopathy and thrombosis of the native vessels.
POSTOPERATIVE COMPLICATIONS
Postoperative complications after liver transplantation can occur for a variety of reasons. The preoperative condition of the patient is strongly associated with the probability of development of a complication. The sicker the patient at the time of transplantation, the greater the chance that there will be a complication and the longer will be the hospital stay. In the following sections, several of these potential complications are discussed.
Primary Nonfunction
Primary nonfunction is a poorly understood complication that occurs in about 5% of grafts. Basically, primary nonfunction is the absence of function of the new liver despite adequate perfusion. The diagnosis is clear in 72 to 96 hours after surgery and is manifest by the presence of hepatic encephalopathy, a prothrombin time greater than 25 seconds and uncorrectable by fresh frozen plasma, and the absence of bile output from the liver. This diagnosis should be considered intraoperatively if there is persistent nonsurgical bleeding that occurs after the revascularization of the graft. Postoperatively, the initial liver enzymes are often high (alanine aminotransferase of 5,000 to 15,000), indicating significant hepatocellular injury. This is a nonrecoverable complication and the patient will die. The only effective treatment is early retransplantation.
Primary Dysfunction
An additional 15% of recipients may suffer from a period of initial dysfunction or prolonged cholestasis. These patients can be differentiated from those with primary nonfunction. Patients with graft dysfunction are not encephalopathic, even though they may have poor bile output or persistently elevated clotting studies. Unfortunately, it usually requires 48 to 72 hours after the transplantation
before the two diagnoses can be separated. Primary dysfunction is related to organ harvest injury. A liver biopsy usually shows disordered hepatic cords with ballooning of the hepatocytes and hepatocyte dropout. The clinical course of these patients is often characterized by a persistent hyperbilirubinemia, despite normalization of the liver enzymes and coagulation factors. Most of these patients eventually normalize their hepatic function, but it may take 3 to 6 months. A few patients remain persistently cholestatic and require eventual retransplantation. Several factors that seem to carry an increased risk for the development of primary dysfunction include prolonged donor hospitalization (greater than 3 days), older age (greater than 49 years), extended preservation time (greater than 18 hours), fatty changes in the donor liver biopsy specimen, reduced-size liver grafts, and a younger recipient age (9). Primary dysfunction can also be difficult to separate from rejection. Primary dysfunction patients should have serial liver biopsies to ensure persistent laboratory abnormalities are not from concomitant rejection.
before the two diagnoses can be separated. Primary dysfunction is related to organ harvest injury. A liver biopsy usually shows disordered hepatic cords with ballooning of the hepatocytes and hepatocyte dropout. The clinical course of these patients is often characterized by a persistent hyperbilirubinemia, despite normalization of the liver enzymes and coagulation factors. Most of these patients eventually normalize their hepatic function, but it may take 3 to 6 months. A few patients remain persistently cholestatic and require eventual retransplantation. Several factors that seem to carry an increased risk for the development of primary dysfunction include prolonged donor hospitalization (greater than 3 days), older age (greater than 49 years), extended preservation time (greater than 18 hours), fatty changes in the donor liver biopsy specimen, reduced-size liver grafts, and a younger recipient age (9). Primary dysfunction can also be difficult to separate from rejection. Primary dysfunction patients should have serial liver biopsies to ensure persistent laboratory abnormalities are not from concomitant rejection.
Vascular Complications
Vascular complications are known to occur with the vena cava, portal vein, and particularly the hepatic artery anastomoses. Complications of the hepatic vein or venal caval anastomoses primarily cause outflow obstruction (10). This obstruction leads to the clinical symptoms of graft swelling, medically uncontrollable ascites, and poor urine output, mimicking Budd-Chiari syndrome. These suprahepatic or infrahepatic caval obstructions are usually caused when either a whole graft that is small for a recipient or a reduced-size graft rotates into the right upper quadrant recess, thereby torquing the caval anastomosis. This can be confirmed in the early postoperative period by abdominal ultrasound. Vena caval obstruction in the early postoperative period requires reoperation for correction. When implanting small whole livers or reduced-size livers, the graft must be rotated 90 degrees to prevent an obstruction. In addition, the technique for triangulating the caval anastomosis has been described to be associated with a reduced incidence of obstruction. Stricture at the anastomotic site, which causes late outflow obstruction, may be documented by venography and pressure measurements, and can usually be treated by balloon dilatation.
Thrombosis of the portal venous anastomoses is an infrequent complication, occurring in 2% to 3% of transplants. This complication is usually technical and occurs particularly when there is a size disparity between vessels, as noted in reduced-sized grafts. The diagnosis is made by abdominal duplex Doppler ultrasound. If the portal vein clots immediately after surgery and is not corrected, the graft undergoes necrosis and is lost. Therefore, prompt reoperation with declotting of the vein and repair of the technical problem that caused the thrombosis is required to salvage the graft (11).
Hepatic artery thrombosis (HAT) is the most frequently encountered vascular complication. The hepatic artery is an “end artery” that supplies nutrition predominantly to the extrahepatic and intrahepatic biliary system. If HAT occurs, several potential problems can follow: rarely, submassive to massive hepatic necrosis with intrahepatic
abscess formation; necrosis of the extrahepatic biliary system with a bile leak; or necrosis of a portion of the intrahepatic biliary system with formation of infected intrahepatic bilomas and biliary strictures. The incidence of HAT in the pediatric age group has been reported to be as high as 25% versus 5% in adult series. At our center, the overall incidence of HAT is 8%.
abscess formation; necrosis of the extrahepatic biliary system with a bile leak; or necrosis of a portion of the intrahepatic biliary system with formation of infected intrahepatic bilomas and biliary strictures. The incidence of HAT in the pediatric age group has been reported to be as high as 25% versus 5% in adult series. At our center, the overall incidence of HAT is 8%.
HAT is a difficult diagnosis to make on clinical grounds alone. The classic presentation of HAT is fever beginning 10 days to 2 weeks posttransplantation. The fever is occasionally associated with parenchymal enzyme changes. Graft enlargement with increased firmness may also be present. Confirmation of the diagnosis requires a hepatic arteriogram that shows the absence of hepatic arterial flow to the liver.
The treatment of HAT has undergone substantial changes since the mid-1980s. Initially, it was believed that the only therapy for HAT was immediate retransplantation. Later, it was discovered that these patients could be effectively treated with percutaneous, transhepatic drainage of the intrahepatic bilomas. This was then followed by transhepatic dilation and stenting of the subsequent intrahepatic biliary strictures. This therapy was effective in preventing the need for retransplantation in most of our HAT patients, but it required multiple transhepatic procedures over the ensuing months to years. The development of transabdominal color duplex Doppler ultrasound allowed for the frequent noninvasive monitoring of the hepatic artery. This has prompted the rethinking of the management of HAT. At our center, we follow hepatic artery flow with daily transabdominal Doppler studies for 2 weeks. If a change occurs in the Doppler signal, the patient immediately undergoes an arteriogram. If the arteriogram shows a thrombosis, then urokinase is directly infused into the artery. This dissolves the clot and restores arterial flow to the liver. The patient then is taken to the operating room, where the hepatic artery is reexplored and the arterial anastomosis is revised, either by simple revision or by improving arterial inflow by adding an iliac arterial graft from the recipient aorta to the donor hepatic artery. We have been using this aggressive approach to HAT since 1992 and have had four children follow this protocol. None of these children has required retransplantation, and only one (the first) has had any biliary complications. The early detection and aggressive treatment of HAT can effectively eliminate the traditional complications associated with it. In addition to close monitoring of the hepatic artery flow, some centers routinely use additional pharmacologic methods to decrease the thrombosis rate. These regimens include one or more of the following: low-dose aspirin, dipyridamole, low-molecular-weight dextran, heparin, and prostaglandin E1. It is not clear if any of these manipulations make a difference because there are no randomized studies. In our pediatric transplants, we only use low-molecular-weight dextran and aspirin in those patients in whom the anastomosis was technically difficult (approximately one-third of the cases). Pharmacologic therapy, however, cannot compensate for poor surgical technique. Several additional factors have been reported to be associated with HAT: cyclosporine use, small recipient size (less than 16 kg), a recipient hepatic artery less than 3 mm, edema of the graft that occurs as a complication of rejection, and a hypercoagulable posttransplantation state that has been associated with diminished protein C, protein S, and antithrombin 3 levels (12,13).
Biliary Complications
The biliary anastomosis in transplantation has been called the Achilles’ heel of the procedure. The reported incidence of biliary complications ranges from 2.3% to 38% (14,15). The best method for biliary reconstruction in the absence of a usable recipient bile duct is the end-to-side Roux-en-Y choledochojejunostomy and, when a suitable recipient bile duct is available, an end-to-end choledocho-choledochochostomy.
In a review of our biliary complications, we noted an overall rate of 14%. There was a large preponderance of strictures, with only 12% of the grafts having bile leaks. Most of the early (less than 1 year posttransplantation) biliary complications were associated with HAT. Our initial approach to the biliary complications related to HAT was retransplantation; however, these patients experienced a 60% mortality and a 20% rethrombosis rate. Our next approach was to deal directly with the biliary complications of intrahepatic bilomas and biliary strictures. This was accomplished using transhepatic drainage of abscesses and transhepatic balloon dilatation of strictures. With this aggressive approach, 64% of the grafts with acute biliary complications from HAT were salvaged, and 50% of these patients remain free of biliary complications. Unfortunately, the transhepatic approach is not a panacea, usually requiring more than one procedure and a duration of therapy ranging from 1 to 14 months. During catheter drainage, the patient is at risk for cholangitis, especially during catheter manipulations or contrast studies. Two patients died in our series from biliary catheter-related gram-negative sepsis. It is imperative that broad-spectrum antibiotics be started before and continued for at least 24 hours after any biliary procedure, including contrast studies.
Little data exist regarding late (more than 1 year posttransplantation) biliary strictures. In our series, late biliary strictures occurred in six (4%) patients; two were in duct-to-duct anastomoses and four were in Roux-en-Y reconstructions. Their onset was insidious, occurring from 18 to 51 months after transplantation, with only one patient presenting with symptoms of biliary stasis. Most of theses patients were initially treated for rejection as outpatients, and further workup with an abdominal ultrasound
was initiated only after they failed to respond to therapy. A mechanical problem with the biliary tract must be considered in the differential diagnosis of late hyperbilirubinemia or transaminase elevations. Strictures of the hepatic duct bifurcation were a particularly difficult management problem. Five such strictures occurred in the early group and two in the late group. A transhepatic approach was unsuccessful in treating all the late strictures and two of the early strictures. Operative revision was performed in three grafts (one early and two late). At each exploration, dense fibrous tissue was noted at the site of the strictures. This type of biliary injury could be explained by either an ischemic insult or trauma to the area (16). However, the long time to the development of these strictures makes an injury that occurred at the time of transplantation unlikely. We use indwelling stents for biliary reconstruction. It has been suggested that the stent could be a source of chronic irritation to the biliary system, causing stricture formation (17).
was initiated only after they failed to respond to therapy. A mechanical problem with the biliary tract must be considered in the differential diagnosis of late hyperbilirubinemia or transaminase elevations. Strictures of the hepatic duct bifurcation were a particularly difficult management problem. Five such strictures occurred in the early group and two in the late group. A transhepatic approach was unsuccessful in treating all the late strictures and two of the early strictures. Operative revision was performed in three grafts (one early and two late). At each exploration, dense fibrous tissue was noted at the site of the strictures. This type of biliary injury could be explained by either an ischemic insult or trauma to the area (16). However, the long time to the development of these strictures makes an injury that occurred at the time of transplantation unlikely. We use indwelling stents for biliary reconstruction. It has been suggested that the stent could be a source of chronic irritation to the biliary system, causing stricture formation (17).
Retained biliary stents deserve additional mention. We have encountered two patients in whom the surgically placed biliary stent failed to pass 4 and 26 months after transplantation. In both cases, the stent served as a nidus for biliary stone formation, resulting in biliary tract obstruction. Both patients required stent removal followed by transhepatic biliary lithotripsy to remove the impacted stones. We have also seen one patient in whom a retained stent perforated the Roux-en-Y limb. If, after 4 months, a stent is still found in either the biliary system or the Roux-en-Y limb, serious consideration should be given to its removal.
Infection
Infection from bacterial, viral, or fungal causes continues to be the single largest complication after liver transplantation. Bacterial infections have predominated at our center. Most of these are related to central lines. We change all intraoperative central lines within 5 days, and if longer central access is necessary, cuffed central lines are inserted. Intraabdominal bacterial infections are not as frequent now as they were in the past. Several factors have contributed to this decrease: intraoperative bacterial and fungal cultures are taken of both subhepatic spaces and patients are immediately treated for any positive bacterial or fungal results; intraabdominal hematomas are surgically evacuated to remove them as a potential culture medium; and fungal cultures of the Roux-en-Y lumen are taken and the patient immediately treated if he or she is positive. Despite these measures, a persistent problem is bacterial and fungal infections at the cut edge of a reduced-size liver graft. All patients with a reduced-size graft have a fluid collection at the cut surface; in our series, 54% (13/24) of these were of a significant size. Of these, 62% (8/13) became infected, and of these, three were fungal. Bacterial infections were readily diagnosed by percutaneous aspiration followed by percutaneous drainage of the abscess. If the abscess at the cut surface contains fungus (Candida sp), operative drainage with Penrose drains is necessary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree