Background
Borderline ovarian tumors are generally diagnosed in young women. Because of the young age of patients at first diagnosis and at recurrence, and given the good prognosis of borderline ovarian tumors, a conservative surgical approach in those women who wish to preserve their fertility is advised. In this scenario, transvaginal ultrasound examination plays a key role in the detection of borderline ovarian tumor recurrence, and in assessment of amount of normal functioning parenchyma remaining. To date, no data are available about the natural history of borderline ovarian tumor recurrence.
Objective
The aim of the study was to determine growth rate of recurrent ovarian cysts by a scheduled follow-up by ultrasound examination, in women previously treated with fertility-sparing surgery due to borderline ovarian tumors.
Study Design
In this prospective observational study, we collected data from 34 patients previously treated with fertility-sparing surgery due to borderline ovarian tumors, who had a suspicious recurrent lesion. The patients underwent transvaginal ultrasonographic examination every 3 months, until the clinical setting recommended proceeding with surgery. According to cyst size at study entry, they were categorized into 3 groups: ≤10 mm, 10-20 mm, and >20 mm. Summary statistics for cyst size, growth rate, and the probability of remaining within the same dimension category at first ultrasound during the follow-up were also obtained. For each cyst the growth rate was calculated as the slope of the linear interpolation between 2 consecutive measurements.
Results
Follow-up timing ( P < .001), cyst size ( P < .001), and micropapillary pattern ( P < .001) were factors significantly affecting the cyst growth both in univariate and multivariate analysis. According to size category at first ultrasound, growth rate ranges from a minimum of 0.06 mm/mo for cysts <10 mm up to 1.92 mm/mo for cysts >20 mm. The final histology of all recurrent lesions confirmed the same histotype of primary borderline ovarian tumors.
Conclusion
This article represents the first observational study that describes the trend in the growth rate of borderline ovarian tumor recurrence in relation to their size detected at the first ultrasound examination. The findings of this study seem to confirm, in selected patients, that a thorough ultrasonographic follow-up of borderline ovarian tumor recurrence has proven to be safe and feasible. The final goal of such management is to maximize the impact on fertility potential of these young women without worsening their prognosis.
Introduction
Borderline ovarian tumors (BOT) account for approximately 10-15% of all ovarian epithelial tumors. These tumors are generally diagnosed in young women, as International Federation of Gynecology and Obstetrics (FIGO) stage I in nearly 70% of the cases, with a 5-year survival rate of 95-97%.
Given the young age at diagnosis and the good prognosis of BOT, a conservative surgical approach in those patients who wish to preserve their fertility is advised. Fertility-sparing surgery (FSS) is defined as the preservation of the uterus and of at least part of 1 ovary with a complete staging procedure. Although this strategy has proven to be safe and feasible, recurrences have been described in 5-56% of cases. Hence, a close follow-up based on scheduled pelvic ultrasounds is mandatory for early identification of any relapse after FSS. At transvaginal ultrasound BOT recurrences can be easily identified, within the residual ovarian parenchyma, as unilocular-solid cysts (in case of serous or endocervical-type mucinous borderline tumors) or multilocular masses (in case of mucinous intestinal-type borderline tumors), mimicking the morphological features of the primary tumor.
In the past, standard treatment of recurrences consisted of definitive ablation of internal genital organs. Nowadays, a repeated FSS can be offered to patients of fertile age. In this setting, transvaginal ultrasound examination plays a primary role in the detection of BOT recurrence, and in the assessment of the amount of normal functioning parenchyma remaining. Sometimes, small lesions can be encased in the ovary and thus not easily recognizable during surgical exploration, furthermore they may not be identified by conventional imaging modality and therefore left behind. However, delaying the schedule of the surgical procedure could let small recurrences protrude on the ovarian surface and microscopic lesions become visible, but no data are available about the natural history of BOT recurrences.
The aim of this study was to determine the growth rate of recurrent ovarian cysts in women previously treated by FSS due to BOT.
Materials and Methods
A prospective observational study was designed. From October 2000 through December 2011, 34 patients previously treated with FSS due to BOT, who had a suspicious recurrent lesion at follow-up transvaginal ultrasound examination, and with no indication to immediate surgical treatment ( Table 1 ), were enrolled in a prospective protocol of longitudinal surveillance. All patients were followed up for at least 3 months before undergoing surgery. In all, 29 patients were recruited in the Preventive Gynecologic Unit at European Institute of Oncology in Milan, and 5 patients in the Gynecologic Oncology Unit of the University of Sacred Heart in Rome. The review board of both departments approved the study protocol.
| Primary BOT | |
|---|---|
| Age at diagnosis, y, median (range) | 29.0 (14.0–50.0) |
| Histology | |
| Serous | 32 (94.1) |
| Mucinous | 2 (5.9) |
| FIGO stage | |
| I | 11 (32.3) |
| II/III without invasive implants | 19 (55.9) |
| II/III with invasive implants | 4 (11.8) |
| Micropapillarity | |
| No | 20 (58.9) |
| Micropapillary aspect | 8 (23.5) |
| Micropapillary | 6 (17.6) |
| Surgery of primary BOT | |
| Unilateral cystectomy | 5 (14.7) |
| Bilateral cystectomy | 11 (32.4) |
| Unilateral oophorectomy | 16 (47.1) |
| Unilateral oophorectomy and contralateral cystectomy | 2 (5.9) |
All the study patients signed informed consent at enrollment.
All recurrences were detected at the planned follow-up ultrasound examinations. These were scheduled every 3 months for the first 2 years after FSS and every 6 months thereafter.
After the diagnosis of suspicious recurrent lesion, the patients underwent transvaginal ultrasonographic examination and serum biomarker dosage (CA125 and CA19.9) every 3 months, until the clinical setting recommended proceeding with surgery.
All examinations were performed by 2 experienced ultrasound examiners, with 15 years of experience in gynecological oncology ultrasound (D.F. and A.C.T.). High-end ultrasound equipment, 5.0- to 9.0-MHz frequency vaginal probes, and 3.5- to 5.0-MHz frequency abdominal probes were employed. Transvaginal and transabdominal scans were subsequently performed on each patient to ensure a complete examination of the entire pelvic and abdominal cavity.
The following parameters were assessed at any transvaginal ultrasound examination: size of the lesion (3 orthogonal diameters), type of mass (unilocular-solid, multilocular, multilocular-solid, solid), presence and number of papillary projections (defined as any solid protrusion into a cyst cavity with a height of ≥3 mm), number of locules, echogenicity of cyst fluid, irregularity of the surface of papillary projections, and presence of solid tissue other than papillary projections. The color content of the papillary projections at power Doppler examination was estimated subjectively by the ultrasound examiner using a color score as described by Timmerman et al (1 = no vascularization; 2 = minimal vascularization; 3 = moderate vascularization, 4 = high vascularization).
The tumor pattern recognition method was used to make a diagnosis of suspicious recurrence. Morphological features suggestive of recurrences of BOT were: unilocular-solid cysts with at least 1 papillary projection for serous or mucinous endocervical-type BOT, and multilocular cyst without papillations for intestinal-type mucinous borderline tumor. Rarely, recurrent lesions appear as unilocular, multilocular-solid cysts with papillary projections, or as solid masses.
Young patients wanting to preserve fertility but with no immediate pregnancy plan were offered a follow-up program if fulfilling the following criteria: no evidence of metastasis, no ascites, maximum diameter of the suspected recurrent lesion <40 mm, presence of healthy ovarian tissue adjacent to the tumor (namely “ovarian crescent sign”), and negative tumor marker (CA125, CA19.9). Evidence of multiple recurrent lesions did not represent an exclusion criterion and all individual lesions were considered for the analysis.
Patients were offered a further FSS when the following criteria were met: desire of pregnancy, patient’s request due to subjective anxiety, tumor markers above the upper normal limit, rapidly increased growth rate of the cyst defined as doubling of tumor dimension in 3 months, and cyst size ≥40 mm. Definitive surgery was performed in case of patients’ choice, no more pregnancy desire due to age, no more evidence of disease-free ovarian tissue, presence of ascites, or detection of peritoneal implants. All the patients enrolled in this study eventually underwent surgery.
Statistical analysis
Patients characteristics, surgical procedures, and cyst ultrasound features were tabulated and summarized using counts and percentages; continuous variables were expressed by mean, median, SD, and range as appropriate. According to cyst size at study entry, they were categorized into 3 groups: <10 mm, 10-20 mm, and >20 mm. Summary statistics for cyst size, growth rate, and the probability of remaining within the same dimension category at first ultrasound during the follow-up were also obtained. To take into account the influence of the cyst size on the growth rate, at each follow-up time, cysts were reallocated to the new size category if their dimension exceeded the limits of the category at the first visit. For each cyst the growth rate was calculated as the slope of the linear interpolation between 2 consecutive measurements.
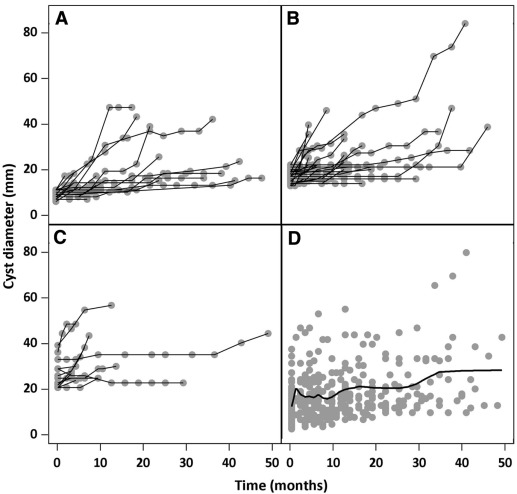
Both univariate and multivariate hierarchical mixed model were constructed to identify determinants of growth rate using time visit as random effect and the log-normal link function. Bayesian information criteria was used for model selection. Cysts were nested within patient. Covariables were age, histology, extraovarian implant, micropapillarity, and number of recurrences. Diameter category (<10 mm, 10-20 mm, and >20 mm) entered the model as a time-dependent covariate. Differences in follow-up durations by diameter at first visit were tested for bias selection using the Kruskal-Wallis test. A panel plot of individual profile growth curves by diameter at baseline was produced ( Figure 1 ). Median time to indication for surgery with 95% confidence interval was estimated by the Kaplan-Meier method. Reaching a cyst size of 40 mm was considered the threshold for surgery. Patients who underwent surgery for reasons other than cyst size upper cut-off were censored. All tests were 2-tailed and considered statistically significant at the alpha level of 0.05.
All analyses were conducted using software (SAS System 9.2 for Windows, SAS Institute Inc, Cary NC).
Materials and Methods
A prospective observational study was designed. From October 2000 through December 2011, 34 patients previously treated with FSS due to BOT, who had a suspicious recurrent lesion at follow-up transvaginal ultrasound examination, and with no indication to immediate surgical treatment ( Table 1 ), were enrolled in a prospective protocol of longitudinal surveillance. All patients were followed up for at least 3 months before undergoing surgery. In all, 29 patients were recruited in the Preventive Gynecologic Unit at European Institute of Oncology in Milan, and 5 patients in the Gynecologic Oncology Unit of the University of Sacred Heart in Rome. The review board of both departments approved the study protocol.
| Primary BOT | |
|---|---|
| Age at diagnosis, y, median (range) | 29.0 (14.0–50.0) |
| Histology | |
| Serous | 32 (94.1) |
| Mucinous | 2 (5.9) |
| FIGO stage | |
| I | 11 (32.3) |
| II/III without invasive implants | 19 (55.9) |
| II/III with invasive implants | 4 (11.8) |
| Micropapillarity | |
| No | 20 (58.9) |
| Micropapillary aspect | 8 (23.5) |
| Micropapillary | 6 (17.6) |
| Surgery of primary BOT | |
| Unilateral cystectomy | 5 (14.7) |
| Bilateral cystectomy | 11 (32.4) |
| Unilateral oophorectomy | 16 (47.1) |
| Unilateral oophorectomy and contralateral cystectomy | 2 (5.9) |
All the study patients signed informed consent at enrollment.
All recurrences were detected at the planned follow-up ultrasound examinations. These were scheduled every 3 months for the first 2 years after FSS and every 6 months thereafter.
After the diagnosis of suspicious recurrent lesion, the patients underwent transvaginal ultrasonographic examination and serum biomarker dosage (CA125 and CA19.9) every 3 months, until the clinical setting recommended proceeding with surgery.
All examinations were performed by 2 experienced ultrasound examiners, with 15 years of experience in gynecological oncology ultrasound (D.F. and A.C.T.). High-end ultrasound equipment, 5.0- to 9.0-MHz frequency vaginal probes, and 3.5- to 5.0-MHz frequency abdominal probes were employed. Transvaginal and transabdominal scans were subsequently performed on each patient to ensure a complete examination of the entire pelvic and abdominal cavity.
The following parameters were assessed at any transvaginal ultrasound examination: size of the lesion (3 orthogonal diameters), type of mass (unilocular-solid, multilocular, multilocular-solid, solid), presence and number of papillary projections (defined as any solid protrusion into a cyst cavity with a height of ≥3 mm), number of locules, echogenicity of cyst fluid, irregularity of the surface of papillary projections, and presence of solid tissue other than papillary projections. The color content of the papillary projections at power Doppler examination was estimated subjectively by the ultrasound examiner using a color score as described by Timmerman et al (1 = no vascularization; 2 = minimal vascularization; 3 = moderate vascularization, 4 = high vascularization).
The tumor pattern recognition method was used to make a diagnosis of suspicious recurrence. Morphological features suggestive of recurrences of BOT were: unilocular-solid cysts with at least 1 papillary projection for serous or mucinous endocervical-type BOT, and multilocular cyst without papillations for intestinal-type mucinous borderline tumor. Rarely, recurrent lesions appear as unilocular, multilocular-solid cysts with papillary projections, or as solid masses.
Young patients wanting to preserve fertility but with no immediate pregnancy plan were offered a follow-up program if fulfilling the following criteria: no evidence of metastasis, no ascites, maximum diameter of the suspected recurrent lesion <40 mm, presence of healthy ovarian tissue adjacent to the tumor (namely “ovarian crescent sign”), and negative tumor marker (CA125, CA19.9). Evidence of multiple recurrent lesions did not represent an exclusion criterion and all individual lesions were considered for the analysis.
Patients were offered a further FSS when the following criteria were met: desire of pregnancy, patient’s request due to subjective anxiety, tumor markers above the upper normal limit, rapidly increased growth rate of the cyst defined as doubling of tumor dimension in 3 months, and cyst size ≥40 mm. Definitive surgery was performed in case of patients’ choice, no more pregnancy desire due to age, no more evidence of disease-free ovarian tissue, presence of ascites, or detection of peritoneal implants. All the patients enrolled in this study eventually underwent surgery.
Statistical analysis
Patients characteristics, surgical procedures, and cyst ultrasound features were tabulated and summarized using counts and percentages; continuous variables were expressed by mean, median, SD, and range as appropriate. According to cyst size at study entry, they were categorized into 3 groups: <10 mm, 10-20 mm, and >20 mm. Summary statistics for cyst size, growth rate, and the probability of remaining within the same dimension category at first ultrasound during the follow-up were also obtained. To take into account the influence of the cyst size on the growth rate, at each follow-up time, cysts were reallocated to the new size category if their dimension exceeded the limits of the category at the first visit. For each cyst the growth rate was calculated as the slope of the linear interpolation between 2 consecutive measurements.
Both univariate and multivariate hierarchical mixed model were constructed to identify determinants of growth rate using time visit as random effect and the log-normal link function. Bayesian information criteria was used for model selection. Cysts were nested within patient. Covariables were age, histology, extraovarian implant, micropapillarity, and number of recurrences. Diameter category (<10 mm, 10-20 mm, and >20 mm) entered the model as a time-dependent covariate. Differences in follow-up durations by diameter at first visit were tested for bias selection using the Kruskal-Wallis test. A panel plot of individual profile growth curves by diameter at baseline was produced ( Figure 1 ). Median time to indication for surgery with 95% confidence interval was estimated by the Kaplan-Meier method. Reaching a cyst size of 40 mm was considered the threshold for surgery. Patients who underwent surgery for reasons other than cyst size upper cut-off were censored. All tests were 2-tailed and considered statistically significant at the alpha level of 0.05.




