Ulcerative Colitis
Terry L. Buchmiller-Crair
Children’s Hospital of New York Presbyterian, Weill Medical College of Cornell University, New York, New York 10021.
Ulcerative colitis (UC) is an idiopathic inflammatory process limited to the colorectal mucosa that is characterized by intestinal inflammation and alteration in bowel function. The overall incidence of UC ranges from 3.5 to 8.0 per 100,000, and is typically seen in adults with a bimodal distribution during the third and eighth decades. The incidence falls to 1.5 to 2.0 per 100,000 for the last two to three decades of life (1). UC is a relatively rare disease in childhood, with only 15% to 20% of patients being diagnosed prior to 16 years of age (2).
The pediatric presentation of UC, however, can portend an ominous clinical course. The principles of medical management parallel those of adults, with the addition of meticulous attention to growth, nutrition, and psychological impact (1). Children with a history of UC for 10 years have an increased risk of developing colon cancer. However, UC is a curable disease if all colorectal mucosa is surgically removed. This initially involved the performance of a total colectomy, proctectomy, and placement of a permanent end ileostomy. However, patient, parental, and surgeon dissatisfaction with the commitment to a lifetime ileostomy led to the development of many contemporary anal-sparing options based on the principle of ileal reservoir creation. The addition of minimally invasive options in the surgeon’s armamentarium has made the surgical treatment of UC an even more attractive option for both the parents of young children, and the body-conscious teenager.
HISTORICAL PERSPECTIVE
Diarrheal disease was described in the early writings of Hippocrates (c. 400 BC) as a significant health problem (4). Bailey, a British pathologist who lived from 1761 to 1823, first reported diarrheal disease attributed to what would later be termed ulcerative colitis (5). He described autopsy reports of intestinal pathology, suggesting that UC was responsible for mortality in the late eighteenth century. It was not until the nineteenth century that a differentiation emerged between the more common infectious diarrhea and UC. Crohn (6), in his historical treatise on UC from 1962, noted autopsy descriptions of 200 cases of diarrhea and dysentery from the Union Army during the Civil War. These cases were likely attributed to UC, and herald the time when the term “ulcerative colitis” was first applied. Wilks and Moxon published their classic anatomic description of UC 16 years later in 1875, differentiating it from dysentery (6). In 1907, Lockhart-Mummery (7) first reported an increased incidence of colon carcinoma in 7 of 36 UC patients. Kirsner (8) later recommended chronic surveillance of UC patients with an illuminated proctosigmoidoscope.
Medical management in this early era consisted mainly of dietary restrictions and homeopathic medications. The original surgical therapy was a sigmoid colostomy described by Pennel in 1850 (9). Subsequently, an irrigation appendicostomy was described in 1902, followed by the use of a completely diverting ileostomy in 1913. These diversions were later used in conjunction with partial resection of the diseased colon with antegrade irrigations initiated either through the appendicostomy or ileostomy.
Total colectomy, with or without a proctectomy, and an end ileostomy were considered definitive therapy in the 1940s. The resultant high-output ileostomy was associated with significant peristomal complication rates until Brooke described surgical maturation of the stoma in 1952 (10). Brooke’s technique permitted effective appliance application, therefore abrogating many of these local complications.
The modern era of surgical therapy for UC was heralded by a novel anus-saving technique using a rectal mucosectomy and intestinal pull-through procedure reported by Ravitch and Sabiston in 1947 (11). However, the resultant acute and chronic postoperative complications
rendered this technique initially unacceptable, being favored by the subsequently developed Kock continent ileostomy. The Koch pouch permitted reasonable fecal continence via the catheterized stoma and potential freedom from a chronic appliance (12). However, a stoma was still necessary, and the procedure was accompanied by significant complications.
rendered this technique initially unacceptable, being favored by the subsequently developed Kock continent ileostomy. The Koch pouch permitted reasonable fecal continence via the catheterized stoma and potential freedom from a chronic appliance (12). However, a stoma was still necessary, and the procedure was accompanied by significant complications.
This undesirable situation led to a reevaluation of the intestinal pull-through procedure initially described by Ravitch and Sabiston. A modification of the Soave technique commonly used for the correction of Hirschsprung’s disease was proposed for the treatment of UC by Martin and LeCoultre (13). Emphasizing extensive preoperative preparation, precise operative technique, and the addition of a temporary diverting ileostomy, this modification resulted in an acceptable complication rate and functional results. However, significant postoperative morbidity persisted with an undesirable high stool frequency rate and nighttime incontinence following ileostomy closure (14).
These complications prompted the further addition of several types of surgically constructed ileal reservoirs or pouches that could be used in conjunction with the endorectal ileal pull-through procedure (ERIPT) (15,16,17,18,19). Indeed, pediatric surgeons were leaders in developing the contemporary surgical options now used in the management of UC. The addition of a pouch offered faster postoperative recovery, less frequent stooling, and facilitated an earlier return to a more normal pattern of daily living. The introduction of an ileal pouch had its negative effect, however, creating a new entity of reservoir inflammation termed pouchitis. Pouchitis manifests as diarrhea, bleeding, and pain, and requires chronic antibiotic therapy in 26% to 40% of patients (18,19,20,21). Pouch use is still debated, because outcomes following the ERIPT with or without a pouch are relatively equivalent after the first year. This has prompted some pediatric surgeons to avoid pouch use in the younger pediatric patients who seem to have less morbidity in the early postoperative period.
ETIOLOGY
The etiology of UC remains undetermined. UC occurs with an equal gender distribution and is four times more frequent in the white population. The incidence of UC is increased in the United States and Europe, especially in Scandinavia and England, demonstrating regional variation (22,23,24). UC also occurs four times more frequently in Jewish populations (25), with a higher incidence in Jewish immigrants to North America and Europe compared with Jews living in Israel. There is a lower incidence in Mediterranean regions, Africa, and Asia. These variations suggest a strong genetic risk for UC coupled with potential environmental and/or socioeconomic factors. Although the incidence of UC exceeded that of Crohn’s disease in the first half of the twentieth century, these inflammatory bowel diseases (IBDs) are now equivalent.
The etiology of IBD has been related to environmental factors, but no conclusive evidence implicates diet, as previously suggested. The effect of cigarette smoking on the incidence of IBD has been widely studied. Harries and colleagues first linked a lower incidence of UC with smoking in 1982 (26). Although smoking doubles one’s risk for Crohn’s disease, the incidence of UC is reduced by 50%. At least one adult case has shown remission of UC related to the use of nicotine gum. The risk of UC remains increased in former smokers as compared with those who have never smoked (27).
Appendectomy appears to protect against the development of UC (28). Patients having undergone prior appendectomy developed symptoms of UC at a significantly later age. However, there was no impact on the extent of disease, disease course, use of immunosuppressive therapy, or ultimate need for surgical treatment. Prior appendectomy is also associated with the development of primary sclerosing cholangitis. The performance of an appendectomy is unlikely to be of benefit in cases of established UC (28).
Genetic Predisposition
A genetic predisposing factor would help explain the geographic, racial, and cultural distribution of UC. Approximately 15% of UC patients have one or more family members with a form of IBD. The relative risk for UC in siblings is 16.6 with a high degree of concordance for disease characteristics (29).
Patients with UC complicated by idiopathic ankylosing spondylitis and uveitis have an increased incidence of the major antigen type HLA-W27 (30). Several other potential candidate genes have been suggested with an association of specific HLA-DRB1 alleles (31) and C3435T MDR1 gene polymorphism (32). Antineutrophilic cytoplasmic antibodies have been detected in healthy family members of UC patients (33). Abnormal plasma polyunsaturated fatty acid patterns are noted during periods of both active and inactive IBD, in both UC and Crohn’s disease (34). Mucin abnormalities have been suggested as a predisposing factor in UC related either to secondary infection or toxin invasion (35).
A two-stage genomewide search for susceptibility loci in IBD was performed involving 186 affected siblings with loci on chromosomes 2 and 6 linked to UC (29). Global gene expression profiles of inflamed colonic tissue by DNA microarray analysis has identified 170 genes with differing expression profiles for IBD (36). Fine mapping may ultimately lead to specific gene identification for UC.
Infectious
A specific infectious organism was originally suspected in IBD. The role of bacteria or viruses as primary pathogens
versus secondary invaders remains unclear. The development of Crohn’s disease may be related to Mycobacterium paratuberculosis (37), although conclusive evidence is still lacking (38). Despite extensive investigation, no specific relationship between an infectious bacterial or viral etiologic factor and UC has been demonstrated.
versus secondary invaders remains unclear. The development of Crohn’s disease may be related to Mycobacterium paratuberculosis (37), although conclusive evidence is still lacking (38). Despite extensive investigation, no specific relationship between an infectious bacterial or viral etiologic factor and UC has been demonstrated.
Immunologic
The immunologic response of the colonic mucosa to either a chemical or bacterial antigen is the most attractive contemporary theory of UC. The clinical spectrum of disease, histological appearance, and changes in immune system function all suggest that an underlying immunocellular response produce the mucosal changes seen in IBD (39). Potential pathogenic factors (dietary, genetic, environmental, and microbiologic) may individually, or jointly, be critical in inciting this immune response. The specific effector cell (plasma cells, T cells, macrophages, or neutrophils), and the role of their secreted products (antibodies, eicosanoids, cytokines, and oxygen radicals) have yet to be fully delineated.
Most investigation has focused on the mucosal T cell, with directed efforts toward elucidating the mechanism of antigen processing by T-cell receptors. The response of the trimolecular complex to the presenting antigen with varying utilization of the α- and β-chain regions of the T-cell receptor and human leukocyte antigen (HLA) surface molecules is being defined. Colonic T cells in the lamina propria produce increased interleukin 5 (IL-5) in UC patients (40). Abnormal utilization of the T-cell receptor has been identified in UC with decreased expression of the Vβ2 genes in the lamina proprial T cells and the Vδ3 genes in intraepithelial lymphocytes (41,42). It is hypothesized that restricted or predominant antigens trigger a specific T-cell response in IBD.
The soluble mediators released by cells during the IBD inflammatory response are being comprehensively studied. Fiocchi (43) provided a review of the complex intestinal mucosal cytokine network in IBD. Interleukin 6 (IL-6) levels are elevated in Crohn’s disease, but not in UC (44). Interleukin 1 (IL-1), a proinflammatory cytokine, and its receptor antagonist (Il-lra), an antiinflammatory protein, show imbalance in the mucosa of IBD patients (45). Tumor necrosis factor-alpha (TNF-α) is increased in both the blood and stool of pediatric IBD patients (46,47). Mediator profiles could potentially be used to differentiate UC from Crohn’s disease. It is hypothesized that correction of mediator imbalance could ultimately have therapeutic implications in IBD (39).
Nonimmune cells, including epithelial, endothelial, muscle, and intestinal fibroblasts, also have a putative role in the pathogenesis of IBD. These nonimmune cells function during antigen presentation and immune regulation, and as secretors of soluble mediators. Gut epithelial cells display and present class II (HLA-DR) antigen (48,49). Epithelial cells produce platelet-activating factor, a potent proinflammatory lipid mediator that occurs in excess in UC (50). Intestinal muscularis mucosa cells and mucosal fibroblasts proliferate in response to immune-derived cytokines, including IL-1, IL-6, and TNF-α (51). It appears, therefore, that the intestinal immune response in IBD involves a complex interaction between immune cells, cytokines, fibroblasts, and epithelial, mesothelial, and endothelial cells. Defining this interaction may hold the key to selective therapeutic ablation of the intestinal IBD response.
PATHOLOGIC AND HISTOLOGIC CHARACTERISTICS
UC and Crohn’s disease are distinct entities with a significantly different clinical prognosis that can be difficult to differentiate using anatomic and histologic criteria. UC is a chronic mucosal inflammation of the large intestine invariably involving the rectum, and extending proximally in continuity to involve all or part of the colon. Children are most likely to develop pancolitis, with the rectosigmoid remaining the most severely involved area. In 10%, there is mild terminal ileal inflammation and edema. This backwash ileitis is a misnomer because no evidence supports the etiologic reflux of luminal contents (52).
Crohn’s disease, in distinction to UC, is a transmural granulomatous disease that typically involves the small intestine. The seldom seen Crohn’s colitis can, however, be initially confused with UC. Frank distal ileal involvement should implicate Crohn’s disease. Cases of indeterminate colitis should be thoroughly investigated prior to undertaking surgical treatment, when possible.
Gross Pathology
The inflammatory response of UC is limited to the colonic mucosa and submucosa. Grossly, the colonic mesentery becomes shortened, with increased superficial vascularity and fat deposition. Endoscopic examination of the lumen reveals a hypervascular and friable mucosa with superficial ulcers that undermine the submucosa in mild, active disease. Advanced disease leads to mucosal fissures with islands of bridging mucosa resulting in the typical “cobblestone” appearance with pseudopolyp formation. These mucosal changes can revert to a more normal nonpolypoid appearance during periods of remission (53). UC typically develops in a circumferential mucosal pattern in contrast to Crohn’s disease that manifests as mesenteric linear ulcerations.
Histology
As noted previously, typical UC manifests as a mucosal-submucosal process, but can appear microscopically to
involve deeper layers. The histologic appearance of UC, unfortunately, cannot always be fully differentiated from infectious and inflammatory conditions, such as shigellosis, amebiasis, and gonorrheal colitis (53). Early, acute UC appears as an infiltrate of round cells and polymorphonuclear leukocytes into the crypts of Lieberkuhn at the mucosal base with resultant characteristic crypt abscesses (Fig. 87-1). The overlying epithelial cells stain poorly and are vacuolated. The ulcerated areas contain collections of collagen and granulation tissue that descend to, but rarely through, the muscularis. Transmission electron microscopy demonstrates swollen mitochondria with widened interstitial spaces, and a broadened endoplasmic reticulum. During periods of remission the mucosa may revert to normal.
involve deeper layers. The histologic appearance of UC, unfortunately, cannot always be fully differentiated from infectious and inflammatory conditions, such as shigellosis, amebiasis, and gonorrheal colitis (53). Early, acute UC appears as an infiltrate of round cells and polymorphonuclear leukocytes into the crypts of Lieberkuhn at the mucosal base with resultant characteristic crypt abscesses (Fig. 87-1). The overlying epithelial cells stain poorly and are vacuolated. The ulcerated areas contain collections of collagen and granulation tissue that descend to, but rarely through, the muscularis. Transmission electron microscopy demonstrates swollen mitochondria with widened interstitial spaces, and a broadened endoplasmic reticulum. During periods of remission the mucosa may revert to normal.
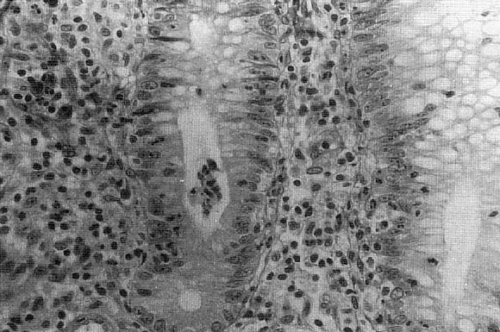 FIGURE 87-1. Micrograph of intestinal crypt with diminished goblet cells and a characteristic crypt abscess. |
With chronic inflammation, the muscularis becomes thickened and fibrotic, with gross flattening of haustral folds. Full thickness intestinal wall inflammation occurs only in fulminant UC and toxic megacolon (30). Chronically inflamed mucosa may become atrophic and is predisposed to dysplasia in long-standing disease. Biopsy confirms UC and assists the clinical picture in assessing disease activity.
CLINICAL PRESENTATION
UC is typically diagnosed in young adults, although 18% of cases present between 10 and 20 years of age, and 4% in children younger than 10 years of age (52). Symptoms associated with pediatric UC are listed in Table 87-1. Presentation is usually insidious, with persistent diarrhea and rectal bleeding, commonly with pus or mucous. In 15% of children, however, the onset is fulminant with profuse bloody diarrhea, severe crampy abdominal pain, fever, and occasionally sepsis. The distribution of UC in children is usually more extensive compared with adults and indices
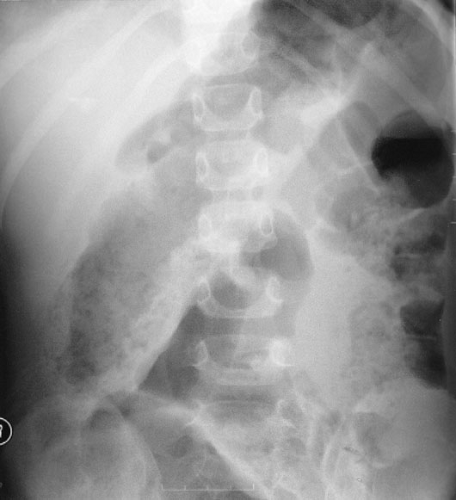
of disease severity can be unreliable. Most children respond to the initiation of medical therapy, but 5% will develop toxic megacolon (Fig. 87-2) necessitating emergent surgery.
of disease severity can be unreliable. Most children respond to the initiation of medical therapy, but 5% will develop toxic megacolon (Fig. 87-2) necessitating emergent surgery.
TABLE 87-1 Clinical Presentation of Pediatric Ulcerative Colitis. | |
|---|---|
|
The significant rectal bleeding that occurs is predictable when the gross and microscopic picture of UC is considered. The mucosal edema and ulcerations combined with the exuberant granulation tissue preclude normal colonic absorption. The resultant diarrhea irritates and produces further disruption of the friable mucosa. Crampy lower abdominal pain or tenesmus can be presenting symptoms, but tend to occur more commonly with chronic disease. Other chronic symptoms include anemia, anorexia, weight loss, growth retardation, and chronic fatigue. Often, these chronic symptoms manifest in children as the lack of desire to participate in social and athletic activities.
Growth retardation is predominantly related to the degree of intestinal inflammation and resultant poor nutrition in combination with the side effects of medical therapy (54). Approximately 14% of pediatric UC patients have decreased height at the time of diagnosis (55). Many more manifest decreased growth velocity, as well as delayed sexual maturation (56). Short stature UC patients have normal growth hormone levels, but lower urinary gonadotropins (57). There is a linear relationship between growth and disease activity, which is independent of steroid use (54). This growth delay persists throughout puberty and is not reversed after surgery, implicating that growth failure is a “prepatterned” manifestation of IBD (54).
Most children develop quiescent colitis punctuated by periodic exacerbations, often precipitated by stress or infections. Although not causative, psychological factors and stress do play a role in relapse rates. Permanent remission after a single attack occurs in fewer than 10% of children with UC. Many progress to chronic colitis with shorter and less frequent remissions. Nearly 50% of those diagnosed in childhood will need a colectomy before the age of 18 (58) as will nearly one-half of all children with pancolitis. Predictive factors for colectomy also include steroid use. Nearly 77% of those who are refractory to medical management came to operation within 3 years. Falcone and colleagues provide a review on predicting the need for colectomy in the pediatric UC patient (58).
Colorectal carcinoma is reported in 3% of children with UC patients within 10 years of disease presentation and increases by 10% to 15% with each subsequent decade (3).
Cancer risk is associated with pancolitis, earlier symptom development, and frequent flares. Dysplasia on endoscopic biopsy indicates a high risk for subsequent carcinoma. Colonoscopic surveillance may be associated with a decreased risk from death from colorectal carcinoma in patients with long-standing UC (59). As the time to dysplasia in IBD may be accelerated, earlier consideration for definitive surgery is encouraged (60).
Cancer risk is associated with pancolitis, earlier symptom development, and frequent flares. Dysplasia on endoscopic biopsy indicates a high risk for subsequent carcinoma. Colonoscopic surveillance may be associated with a decreased risk from death from colorectal carcinoma in patients with long-standing UC (59). As the time to dysplasia in IBD may be accelerated, earlier consideration for definitive surgery is encouraged (60).
Extracolonic Manifestations
Extracolonic manifestations of UC are listed in Table 87-1 and occur in up to 60% of children. Esophagogastroduodeoscopy (EGD) reveals esophagitis and mild to moderate gastritis in 50% of children (61). Gastroduodenal biopsy can show ulcerations, villous atrophy, and increased intraepithelial lymphocytes (62,63). Focally enhanced gastritis, classically considered a marker of Crohn’s disease, also occurs in 20% of patients with UC (61). Liver disease, as manifest by abnormal liver function tests (LFTs), can result from either fatty infiltration or sclerosing cholangitis (64). Although primary sclerosing cholangitis occurs in 15% of adults, it is very uncommon in children (60).
Arthralgia and arthritis occur in 30% of patients and may be the presenting symptom of UC. Joints commonly affected include the wrists, knees, and ankles. Symptoms often respond to systemic steroids, but tend to recur upon medication withdrawal. Symptoms are often independent from the course of underlying colitis.
Skin lesions (65) include both erythema nodosum and pyoderma gangrenosum. Erythema nodosum occurs mainly on the trunk and occasionally limbs and is characterized by tender, red subcutaneous nodules. Pyoderma gangrenosum, a chronic deep ulceration of the skin, occurs most often on the lower limbs. Less than 5% of lesions are resistant to local and systemic therapy. Unlike the arthritides, resistant skin lesions resolve with the remission of colitis or with surgical extirpation of disease.
Children with IBD have decreased bone mineral density that manifests as osteoporosis and osteomalacia. These bone mineralization disorders are predicted by both the cumulative corticosteroid dose and overall nutritional status, especially in girls (66,67). Chronic recurrent osteomyelitis in IBD is very rare (68). Additional conditions include nephrolithiasis in 8% of children, likely related to chronic fluid loss and poor oral intake (30), anemia from hematochezia, uveitis (less than 2%), stomatitis, peripheral thrombotic events, and the rare, acute cerebrovascular accident (69).
Chronic and severe UC can produce emotional changes with feelings of inferiority and depression in the pediatric patient. Although emotional stress has never been established as an etiologic factor in UC, disease exacerbation can occur in association with episodes of severe emotional stress. Care providers should be cognizant of these occasionally subtle changes, and seek professional help for both the child and family.
DIAGNOSIS
Clinical Examination
Children with UC in remission or with only mild active disease may have a normal physical examination. Those with an acute flare may have fever, signs of dehydration, and systemic toxicity. Abdominal tenderness may be elicited on palpation of the left lower quadrant over the sigmoid; abdominal distension is variable. External hemorrhoids may result from frequent defecation due to diarrhea. Digital rectal examination may reveal grossly bloody or heme-positive stool. Anal fissures, fistula, and abscesses are exceedingly rare and would cause one to reconsider the diagnosis in favor of Crohn’s disease. Patients with chronic UC may have decreased height, delayed sexual characteristics, anemia, pallor, and Cushingoid features from chronic steroid use. As the symptoms and physical findings of new-onset UC can be nonspecific, stool specimens should be obtained to exclude a specific responsible infectious pathogen. Screening should include Salmonella, Shigella, and Campylobacter cultures, and analyses for Clostridium difficile toxin and Entamoeba histolytica.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree