Background
Mucosal immunity of the female genital tract plays a critical role in defense against sexually transmitted infections like HIV. Pregnancy is associated with both structural and immunologic alterations in the genital mucosa, but the impact of these changes on its ability to suppress HIV infection is unknown. Current epidemiologic data are conflicting as to whether pregnancy increases the risk of HIV acquisition.
Objective
The purpose of this study was to define the association between antimicrobial peptides and chemokines in cervicovaginal secretions and in vitro HIV infectivity among pregnant and nonpregnant women.
Study Design
Forty pregnant and 37 nonpregnant women were enrolled in a prospective longitudinal cohort study at a single tertiary care women’s hospital in Providence, RI. Cervicovaginal lavage was performed at each study visit. For pregnant women, study visits occurred once per trimester, and there was an optional postpartum visit. For nonpregnant women, study visits occurred across a single cycle that was timed to occur in the proliferative, ovulatory, and secretory phases based on the presumption of a regular menstrual cycle. The impact of cervicovaginal lavage on HIV infectivity was evaluated using a TZM-bl assay and compared between pregnant and nonpregnant women for each visit. The previously validated TZM-bl assay, which uses a luciferase reporting gene to indicate HIV infection of TZM-bl cells, was measured with a luminometer with higher relative light units that indicate greater levels of in vitro HIV infection. Immune mediators were measured with a multiplex bead assay. HIV infectivity and median concentration of each mediator were compared between pregnant and nonpregnant groups with the Wilcoxon rank sum test.
Results
Cervicovaginal fluid from pregnant and nonpregnant women significantly decreased HIV infectivity in both groups compared with positive control (virus only; P <.01), but infectivity was not different between groups ( P ≥.44). During the second and third trimesters, pregnant women experienced suppression of several cervicovaginal immune mediators that included human beta defensin-2; lactoferrin; macrophage inflammatory protein-3α; regulated on activation, normally T-cell expressed and secreted; and stromal cell-derived factor-1 (all P ≤.05). The antimicrobial peptide elafin was significantly correlated with HIV infectivity in both groups across all visits, except at the postpartum visit in the pregnant group (n=16). Secretory leukocyte protease inhibitor also was correlated significantly with infectivity across all visits, but in nonpregnant women only ( P ≤.03).
Conclusion
Cervicovaginal secretions from both pregnant and nonpregnant women contain immune mediators that are associated with HIV infectivity in an in vitro assay; however, infectivity was not different between pregnant and nonpregnant groups. If pregnant women are at increased risk for HIV infection, it is unlikely to be mediated by alterations in the effectiveness of these protective secretions.
HIV/AIDS is the leading cause of death worldwide for women of reproductive age, affecting 1.5 million pregnancies in 2013, which is a statistic that has not improved since 2009. Additionally, there were 240,000 new infections reported in 2013, largely as a result of perinatal transmission. The disproportionate impact of HIV on young women may stem not only from social inequality but also from biologic patterns of heterosexual transmission. Studies of HIV discordant couples consistently demonstrate higher odds ratios of male-to-female transmission than female-to-male transmission, because of the relative anatomic and histologic susceptibilities of the female genital tract to HIV acquisition.
It is important to consider how the susceptibility of the lower genital tract to HIV acquisition may be altered in pregnancy, because the risk of perinatal transmission magnifies the impact of HIV on reproductive-age women. Although several studies have demonstrated high a incidence of HIV infection in pregnant women, data from prospective studies that have compared HIV acquisition in pregnant and nonpregnant women have been conflicting. In a cohort study of >10,000 women in Rakai, Uganda, Gray et al showed that the risk of HIV acquisition doubles during pregnancy, based on an incidence rate of 2.3 per 100 person-years in pregnancy compared with 1.1 per 100 person-years in nonpregnant women. This study was unique in its ability to establish this association after controlling for sexual behaviors in both men and women, which indicated that there may be a physiologic basis to the increased risk of HIV acquisition in pregnancy. However, a more recent meta-analysis reported that there are no significant differences in the risk of HIV acquisition between pregnant and nonpregnant women based on pooled data from 5 prospective cohort studies in African populations.
The first line of defense against HIV acquisition in the female genital tract is the mucosal lining of the epithelial barrier. There is evidence in the literature of pregnancy-mediated alterations in the cytokines, chemokines, and antimicrobial peptides (AMPs) that contribute to genital immunity, but the impact of these changes on HIV infectivity is not known. Studies in nonpregnant women have associated macrophage inflammatory protein (MIP)-3α, RANTES (regulated upon activation, normally T-cell expressed and secreted), human beta defensin (HBD)-2, elafin, and several other immune mediators with protection against HIV. In a previous study, we demonstrated, with a TZM-bl indicator assay, in vitro anti-HIV-1 activity in cervicovaginal secretions from both pregnant and nonpregnant women. This study used the TZM-bl assay that is preferred by the World Health Organization as the most standardized way to measure HIV infectivity to test cervicovaginal lavage (CVL) from pregnant women. The TZM-bl assay uses a luciferase reporting gene to indicate HIV infection of TZM-bl cells. The luminescence is measured with a luminometer, with higher relative light units (RLUs) that indicate greater levels of in vitro HIV infection. The objective of the current study was to use this novel in vitro method to compare, in an adequately powered study, the risk of HIV infectivity between pregnant (1st, 2nd, and 3rd trimester) and nonpregnant women and to investigate the associations among HIV infectivity, pregnancy, and various immune mediators in CVL.
Materials and Methods
Patients and study design
This prospective cohort study was conducted at a single tertiary care women’s hospital in Providence, RI. From 2010–2013, we enrolled HIV-negative pregnant and nonpregnant women between the ages of 18 and 35 years in this longitudinal study. Pregnant women with gestational age of <14 weeks (by best obstetric estimate) and nonpregnant women with at least 3 months of regular menses were offered enrollment. All participants provided written, informed consent during enrollment. Institutional review board approval was provided by the Women & Infants Hospital Institutional Review Board.
Conditions that might alter the risk of HIV infectivity or pregnancy outcomes were criteria for exclusion. These included pregestational diabetes mellitus, chronic hypertension, recent antibiotic use, use of hormonal contraception, current or planned cerclage, planned termination of pregnancy, known fetal anomalies, recent sexually transmitted infection, or recent symptomatic vaginal discharge. Patients at our facility have HIV testing performed in an opt-out manner at the first prenatal visit. Study participants were asked to refrain from using intravaginal products or participating in sexual intercourse without a condom within 48 hours of study visits; those who did not comply were asked to return at a later date.
Study visit protocol
For the pregnant group, study visits occurred once per trimester (<14, 14–28, and after 28 weeks of gestation) followed by an optional postpartum visit at 4–8 weeks after delivery. For the nonpregnant group, visits occurred across the menstrual cycle such that there was 1 visit in each of the proliferative (days 7–10), ovulatory (days 12–16), and secretory (days 20–26) phases. Demographic and clinical data were collected at each visit, and pregnancy outcome data were abstracted from medical records after delivery.
At each visit, participants underwent CVL as previously described. Before CVL, secretions from the posterior fornix were collected and rolled onto a dry slide for Gram stain to test for bacterial vaginosis by Nugent score. After collection, CVL samples were centrifuged at 1500 g for 10 minutes, and supernatant was stored at –80°C until TZM-bl assay performance.
Assessment of HIV inhibition by CVL
The anti-HIV activity of CVL from pregnant and nonpregnant women was assessed with a TZM-bl inhibition assay. Infection is measured by expression of reporter genes under the control of an HIV-1 long terminal repeat. The HIV-1 strain that was used in this assay was X4-tropic, NL4.3. TZM-bl cells were prepared and maintained as previously described.
TZM-bl cells were seeded at 2×10 4 cells per well in a 96-well microtiter plate and allowed to achieve confluence overnight at 37°C. CVL from each patient was diluted 1:4 in TZM-b media and incubated with HIV for 1 hour at 37°C in a final volume of 100 μL. Media was aspirated from the TZM-bl cells and replaced with the mixture of CVL and virus (100 μL) plus 100 μL of TZM-bl media. Viability of TZM-bl cells on treatment with CVL was quantified with the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI) according to manufacturer’s instructions.
The light intensity of each well was measured with the use of a luminometer and was expressed as RLUs. Uninfected TZM-bl cells in media only and cells in CVL only were used as negative controls and for determination of background luminescence. Cell viability was confirmed for each assay. TZM-bl cells that were incubated with HIV-1 alone were used as a positive control, and the median RLU of these wells was set to 100% after the subtraction of background luminescence. Percent inhibition by CVL was calculated by taking the RLU values of CVL-treated infected cells after the subtraction of background luminescence and expressing them as percentages of the RLU values of the positive control.
Measurement of immune mediators
Concentrations of several AMPs and chemokines that have been correlated previously with HIV infectivity were measured in CVL of pregnant and nonpregnant women by multiplex bead assay. This assay used magnetic fluorescent microspheres (Luminex Corp, Austin, TX) coupled to capture antibodies as previously described. Specific capture and detection antibodies and standards for each AMP were sourced as described by Boesch et al. A master mix of antibody-coupled microspheres was prepared by the combination of individual bead sets at a concentration of 500 microspheres for each antibody in a 10-μL final volume in LL-37 ELISA kit dilution buffer (Hycult Biotech, Plymouth Meeting, PA) for each well. Forty microliters of CVL sample or standard followed by 10 μL of the microsphere master mix was placed in each well (Greiner Bio-One, Monroe, NC). CVL samples were diluted in assay buffer when necessary. After the addition of the samples, the plate was incubated overnight (Biotek, Winooski, VT). Fluorescent signal was detected as previously described, with the use of biotinylated detection antibodies with streptavidin-R-phycoerythrin (Prozyme, Hayward, CA) as the secondary detection agent. When the fluorescent signal was too low to measure, the lowest value on the standard curve for each mediator was substituted.
Sample size determination
The sample size was calculated with RLU infectivity estimates from preliminary work. Based on these estimates, we anticipated that the mean RLU in nonpregnant women was expected to be 1.5 million (±SD=1 million). The sample size was calculated with the use of between-factors repeated measures multivariate analysis of variance. The correlation between repeated measures was set at 0.25. To detect a doubling of the anticipated RLU, a sample size of 35 women per group was required, using an α=.01 to account for subgroup analyses and power of 80%. Ten percent over-enrollment was planned a priori to account for anticipated incomplete data collection.
Statistical analysis
Categoric demographic variables were compared between pregnant and nonpregnant groups by Chi-square or Fisher’s exact test. Normally distributed continuous variables were compared by t -test. RLU values, percent inhibition of HIV by CVL, and immune mediator concentrations were not normally distributed, even after logarithmic or other standard data transformations. Therefore, these variables were examined by nonparametric statistical tests instead of repeated measures analysis of variance and other parametric tests. Percent inhibition of HIV by CVL and concentrations of immune mediators were compared between groups at each visit with the Wilcoxon rank sum test. Spearman rank correlations between percent HIV inhibition and concentration of immune mediators were calculated within each group. After stratification by group, percent inhibition and immune mediator concentrations were compared across visits by the Friedman test. Two-tailed probability values of <.05 were considered statistically significant. The impact of a comparison of several immune markers on the statistical test results was assessed by the Benjamini-Hochberg method to adjust the critical value for the declaration of statistical significance while controlling the false discovery rate at 0.05 for each study time point. Statistical analysis was performed with SAS software (version 9.2; SAS Institute, Cary, NC) and GraphPad Prism 6 (GraphPad Software Inc, La Jolla, CA).
Results
Patient and sample characteristics
A total of 77 patients, 40 pregnant and 37 nonpregnant, were enrolled in this prospective cohort. The groups were similar in most demographic characteristics, although pregnant women were more likely to be married and less likely to consume alcohol ( Table 1 ).
| Demographic characteristic | Pregnant (n=40) | Nonpregnant (n=37) | P value |
|---|---|---|---|
| Age, y | .84 a | ||
| Mean±SD | 26.5±5.1 | 26.3±4.6 | |
| Range | 18.0–35.0 | 18.0–35.0 | |
| Mean bodyweight index at first study visit, kg/m 2 ±SD | 27.0±5.3 | 27.8±7.3 | .59 a |
| Nugent score at enrollment, median (range) | 1.0 (0–10) | 3.5 (0–10) | 0.1 |
| Race, n (%) | .11 b | ||
| White | 25 (62.5) | 15 (40.5) | |
| Black | 6 (15.0) | 10 (27.0) | |
| Asian | 0 | 2 (5.4) | |
| American Indian | 1 (2.5) | 0 | |
| Hispanic or Latina | 7 (17.5) | 10 (27.0) | |
| Other | 1 (2.5) | 0 | |
| Marital status, n (%) | .0005 b | ||
| Single | 14 (35.0) | 27 (73.0) | |
| Living with partner | 5 (12.5) | 2 (5.4) | |
| Married | 21 (52.5) | 6 (16.2) | |
| Divorced | 0 | 2 (5.4) | |
| Education level | .12 c | ||
| Some high school | 0 | 2 (5.4) | |
| High school graduate/general education diploma | 14 (35.0) | 9 (24.3) | |
| 1–3 Years college | 12 (30.0) | 9 (24.3) | |
| College graduate | 6 (15.0) | 13 (35.1) | |
| Graduate work | 8 (20.0) | 4 (10.8) | |
| Employment status | .67 b | ||
| Unemployed | 14 (35.0) | 11 (29.7) | |
| Full-time | 20 (50.0) | 16 (43.2) | |
| Part-time | 6 (15.0) | 8 (21.6) | |
| Other | 0 | 1 (2.7) | |
| Unknown | 0 | 1 (2.7) | |
| Annual family income, n (%) | .14 c | ||
| <$10,000 | 6 (15.0) | 6 (16.2) | |
| $10,000–24,999 | 3 (7.5) | 11 (29.7) | |
| $25,000–49,999 | 8 (20.0) | 8 (21.6) | |
| $50,000–74,999 | 10 (25.0) | 6 (16.2) | |
| ≥$75,000 | 7 (17.5) | 4 (10.8) | |
| Unknown | 6 (15.0) | 2 (5.4) | |
| Insurance, n (%) | .10 b | ||
| Private | 18 (45.0) | 21 (56.8) | |
| Medicaid | 21 (52.5) | 11 (29.7) | |
| Uninsured | 1 (2.5) | 4 (10.8) | |
| Other | 0 | 1 (2.7) | |
| Average drinks/wk (past 3 mo), n (%) | .01 b | ||
| 0 | 36 (90.0) | 23 (62.2) | |
| 1–3 | 3 (7.5) | 11 (29.7) | |
| >3 | 1 (2.5) | 3 (8.1) | |
| Tobacco use: packs/d (past 3 mo), n (%) | .87 b | ||
| 0 | 36 (90.0) | 33 (89.2) | |
| <One-half | 3 (7.5) | 2 (5.4) | |
| >One-half | 1 (2.5) | 2 (5.4) | |
| Marijuana use: times/wk (past 3 months), n (%) | .67 b | ||
| 0 | 38 (95.0) | 34 (91.9) | |
| 1–3 | 2 (5.0) | 3 (8.1) | |
| No. of times pregnant, median (range) | 2 (0–6) | 0 (0–5) | .0004 d |
Vaginal swabs were collected at each visit for pH measurement and microscopic evaluation for presence of yeast, bacterial vaginosis, and trichomonads. There were no significant differences between groups in mean Nugent scores or in the number of participants with bacterial vaginosis at each visit. CVL samples were assessed in a reference laboratory for the presence of various immune cells. Numbers of neutrophils, lymphocytes, eosinophils, and basophils did not differ between groups, but nonpregnant women had significantly higher numbers of macrophages in their CVL during enrollment ( P =.04) and first return visit ( P =.05).
HIV inhibition by cervicovaginal fluid
HIV infectivity of TZM-bl cells was reduced significantly in the presence of CVL from both pregnant and nonpregnant women at all visits ( P <.01) and did not differ between groups at any visit ( Figure ). Median percent inhibition of HIV by CVL from pregnant women ranged from 87.0% in the third trimester to 92.9% after delivery and did not change significantly between trimesters ( Supplementary Table 1 ). Percent inhibition of HIV in the nonpregnant group ranged from 83.9% in the ovulatory phase (return visit 1) to 87.5% in the secretory phase (return visit 2). There was no appreciable difference in the ability of CVL to inhibit HIV across phases of the menstrual cycle.
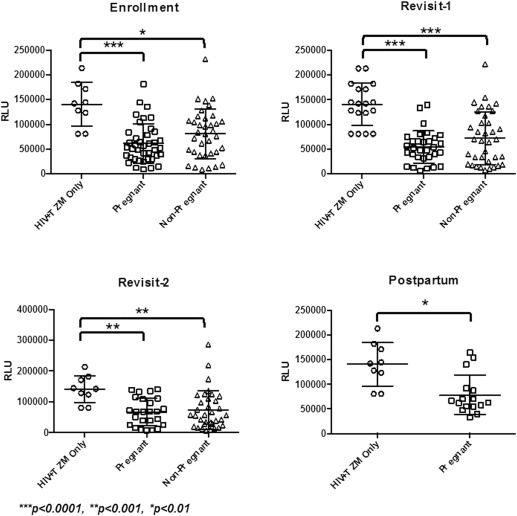
Immune mediators across pregnancy and the menstrual cycle
Concentrations of AMPs and chemokines that previously were shown to impact HIV infectivity were measured in CVL samples ( Table 2 ). In this cohort, we observed a generalized suppression of immune mediators during pregnancy that was most apparent during the second and third trimesters. Concentrations of most mediators were similar between groups at enrollment, which occurred during the first trimester of pregnancy or proliferative stage of the menstrual cycle for nonpregnant women. Macrophage inflammatory protein-3α (MIP-3α) was the only mediator that was suppressed during the first trimester of pregnancy ( P =.001).
| Immune mediator | Enrollment (n=76) a | Return visit 1 (n=67) a | Return visit 2 (n=58) a | Return visit 3 (n=16) a |
|---|---|---|---|---|
| Elafin | ||||
| Pregnant b | 879 (173–4241) | 581 (140–4457) | 631 (144–3872) | 769 (176–7535) |
| Nonpregnant b | 1215 (182–14661) | 958 (210–8308) | 1138 (197–5072) | |
| P value | .2 | .4 | .06 | |
| Human beta defensin-2 | ||||
| Pregnant b | 1292 (428–5828) | 1361 (291–7411) | 1077 (189–9999) | 1455 (234–5896) |
| Nonpregnant b | 1758 (8–14298) | 1914.50 (512–16246) | 1840 (462–7596) | |
| P value | .3 | .03 | .03 c | |
| Human beta defensin-3 | ||||
| Pregnant b | 89 (6–2000) | 51 (6–264) | 38 (5–563) | 6 (6–171) |
| Nonpregnant b | 39 (6–484) | 85 (5–1884) | 95 (6–414) | |
| P value | .1 | .1 | .04 | |
| Lactoferrin | ||||
| Pregnant b | 51 (7–3239) | 40 (12–4209) | 26 (12–7318) | 56 (12–853) |
| Nonpregnant b | 73 (8–697) | 79 (8–1000) | 101 (12–1766) | |
| P value | .2 | .05 | .001 c | |
| LL-37 | ||||
| Pregnant b | 88 (12–3396) | 74 (12–1757) | 53 (12–3563) | 89 (12–1382) |
| Nonpregnant b | 77 (12–1502) | 99 (12–810) | 168 (12–1611) | |
| P value | .7 | .3 | .003 c | |
| Macrophage inflammatory protein-3α | ||||
| Pregnant b | 8 (5–68) | 8 (6–73) | 8 (6–37) | 8 (6–83) |
| Nonpregnant b | 8 (7–741) | 8 (5–1424) | 8 (6–401) | |
| P value | .001 c | .05 | .006 c | |
| Regulated upon activation, normally T-cell expressed and secreted | ||||
| Pregnant b | 6 (6–47) | 6 (4–60) | 6 (5–103) | 6 (4–57) |
| Nonpregnant b | 6 (6–1063) | 6 (6–242) | 6 (4–54) | |
| P value | .06 | .04 | .03 | |
| Stromal cell-derived factor 1 | ||||
| Pregnant b | 13 (3–152) | 12 (4–174) | 9 (4–449) | 15 (3–183) |
| Nonpregnant b | 14.50 (4–378) | 24 (3–2000) | 36 (4–873) | |
| P value | .6 | .04 | .003 c | |
| Secretory leukocyte protease inhibitor | ||||
| Pregnant b | 768 (181–14222) | 960 (100–15786) | 1328 (142–15444) | 1152 (115–8574) |
| Nonpregnant b | 1501 (69–14144) | 1360 (278–16000) | 1585 (178–11212) | |
| P value | .2 | .1 | .4 |
a Pregnancy visits: enrollment, <14 weeks gestation; return visit 1, 14-28 weeks gestation; return visit 2, >28 weeks gestation. Nonpregnant visits: enrollment, proliferative phase; return visit 1, ovulatory phase; return visit 2, secretory phase
b Data are given as median picograms per milliliter (range)
c Statistically significant when controlled for a false discovery rate of 5% for each time point.
Several immune mediators were attenuated progressively throughout the second and third trimesters of pregnancy. During the second trimester (and ovulatory phase of the menstrual cycle for nonpregnant women), human beta defensin (HBD)-2 ( P =.03), lactoferrin ( P =.05), RANTES ( P =.04), stromal cell-derived factor 1 (SDF-1; P =.04), and MIP-3α ( P =.05) levels varied significantly in comparison with the nonpregnant group. Median concentrations of most of these AMPs dropped from the first to the second trimester in pregnant women, while increasing from enrollment to the first return visit in their nonpregnant counterparts. Levels of HBD-2, for instance, dropped significantly from the second trimester to the third within the pregnant group ( P =.03). Data from the third trimester showed an even greater reduction of some of these mediators in pregnancy and significant suppression of HBD-3 ( P =.04) and LL-37 ( P =.001). Levels of HBD-3 decreased significantly throughout pregnancy ( P =.006). CVL concentrations of: secretory leukocyte protease inhibitor (SLPI) and elafin did not differ significantly between groups at any point during the study. After adjustment for multiple comparisons, differences remained statistically significant for MIP-3α in the first trimester (proliferative phase for nonpregnant women) and MIP-3α, lactoferrin, LL-37, SDF-1, and HBD-2 in the third trimester (secretory phase for nonpregnant women).
Association of immune mediator concentrations in CVL with HIV Infectivity
Associations between HIV infectivity and CVL concentrations of immune mediators at each visit were assessed by Spearman rank correlation ( Table 3 ). Concentrations of the AMP elafin were correlated significantly with HIV infectivity in both pregnant and nonpregnant groups throughout all visits, except after delivery. SLPI was associated with HIV infectivity throughout all phases of the menstrual cycle in nonpregnant women, but only in the second trimester in pregnant women. HBD-2 was also associated with HIV infectivity in this group, but only during the second (ovulatory phase) and third (secretory phase) return visits. In pregnant women, HIV infectivity was correlated negatively with LL-37 in the second trimester, HBD-3 in the third trimester, and lactoferrin and SDF-1 in both second and third trimesters. Lactoferrin was also associated negatively with HIV infectivity in the nonpregnant group, but at enrollment (proliferative phase) only. At the pregnant postpartum visit (n=16), previously observed correlations between infectivity and immune mediators were lost. However, associations between infectivity and CVL concentrations of HBD-2 and RANTES were observed for the first time in the pregnant group at this visit. When adjusted for multiple comparisons, correlations between inhibition of HIV infectivity and elafin, lactoferrin, and SDF-1 remained significant for pregnant women in the second and third trimesters. For the nonpregnant group, all proliferative phase correlations remained statistically significant, whereas only elafin and HBD-2 remained to be correlated significantly with infectivity for the ovulatory and secretory phases, respectively.
| Immune mediator a | Enrollment (n=76) b | Return visit 1 (n=67) b | Return visit 2 (n=58) b | Return visit 3 (n=16) b |
|---|---|---|---|---|
| Elafin | ||||
| Pregnant | –0.361 | –0.535 | –0.485 | 0.065 |
| P value | .022 | .002 c | .014 c | .812 |
| Nonpregnant | –0.437 | –0.480 | –0.390 | |
| P value | .008 c | .003 c | .025 | |
| Human beta defensin-2 | ||||
| Pregnant | 0.271 | –0.189 | 0.194 | 0.506 |
| P value | .090 | .309 | .353 | .046 |
| Nonpregnant | –0.275 | –0.353 | –0.485 | |
| P value | .104 | .035 | .004 c | |
| Human beta defensin-3 | ||||
| Pregnant | –0.005 | 0.305 | 0.404 | 0.242 |
| P value | .977 | .096 | .045 | .367 |
| Nonpregnant | –0.159 | –0.103 | 0.005 | |
| P value | .355 | .552 | .978 | |
| Lactoferrin | ||||
| Pregnant | 0.228 | 0.466 | 0.548 | 0.159 |
| P value | .157 | .008 c | .005 c | .556 |
| Nonpregnant | 0.431 | 0.256 | –0.039 | |
| P value | .009 c | .132 | .830 | |
| LL-37 | ||||
| Pregnant | 0.248 | 0.361 | 0.360 | 0.033 |
| P value | .122 | .046 | .077 | .903 |
| Nonpregnant | 0.186 | 0.055 | –0.070 | |
| P value | .279 | .748 | .699 | |
| Macrophage inflammatory protein-3α | ||||
| Pregnant | 0.053 | 0.012 | 0.280 | –0.072 |
| P value | .748 | .950 | .175 | .790 |
| Nonpregnant | 0.165 | 0.231 | 0.203 | |
| P value | .337 | .175 | .257 | |
| Regulated upon activation, normally T-cell expressed and secreted | ||||
| Pregnant | 0.185 | 0.235 | 0.040 | –0.553 |
| P value | .253 | .203 | .851 | 0.026 |
| Nonpregnant | 0.059 | 0.088 | 0.064 | |
| P value | .732 | .608 | .725 | |
| Stromal cell-derived factor 1 | ||||
| Pregnant | 0.190 | 0.531 | 0.526 | 0.261 |
| P value | .240 | .002 c | .007 c | .328 |
| Nonpregnant | 0.177 | 0.061 | –0.084 | |
| P value | .301 | .722 | .642 | |
| Secretory leukocyte protease inhibitor | ||||
| Pregnant | –0.159 | –0.374 | –0.093 | 0.256 |
| P value | .328 | .038 | .658 | .339 |
| Nonpregnant | –0.447 | –0.363 | –0.391 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


