The Chest Wall
Charles N. Paidas
Paul M. Colombani
Department of Surgery, Johns Hopkins University School of Medicine, Johns Hopkins Hospital, Baltimore, Maryland 21287.
Department of Surgery, Johns Hopkins University School of Medicine, Johns Hopkins Hospital, Baltimore, Maryland 21287.
Chest wall deformities comprise a small percentage of congenital anomalies. Much has been written but little concluded from principally retrospective reviews of various treatments for correction of pectus excavatum. For more than 90 years, opinion and guidelines, rather than standards, form the basis of why surgeons repair the excavatum deformity. This anomaly and its mirror image, pectus carinatum, represent the majority of chest wall defects seen in a pediatric surgical practice. The remaining congenital defects of the chest wall include bifid sternum, sternal clefts, Poland syndrome, the Pentalogy of Cantrell, and Jeune syndrome (acquired and congenital). Tumors of the chest wall include enchondroma, rhabdomyosarcoma, Ewing’s sarcoma, osteosarcoma, primitive neuroectodermal tumor (PNET), and a myriad of metastatic tumors finding their way into bone.
CHEST WALL EMBRYOLOGY AND CLINICAL CORRELATIONS
Chest wall development begins on day 35 postfertilization with the formation of ribs, which form from costal processes of the developing thoracic vertebrae. The ribs first develop as cartilaginous precursors that eventually ossify by a process called endochondral ossification (1). Like other regions, the bony skeleton is formed first followed by the skeletal musculature and its corresponding nerves. The sternum forms by the end of the sixth week postfertilization from paired condensations of two mesenchymal structures of the ventral body wall. These bars eventually fuse and chondrify to form a single sternal plate in a craniocaudal direction. Ossification occurs by 60 days postfertilization. The xiphoid process ossifies at birth.
Sternal Clefts
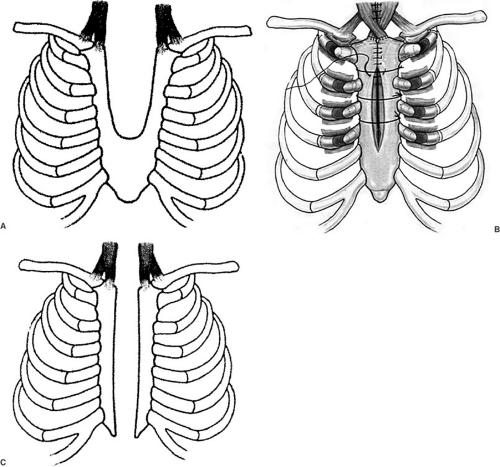
Sternal clefts are the result of midline fusion defects of the mesodermal-derived vertical bars. A spectrum of clefts occurs, depending on the lack of fusion. The most common type of sternal cleft occurs in the upper one-third to one-half of the sternum (Fig. 57-1A). Primary closure, partial resection of upper chest costal cartilages with disruption of the sternoclavicular junction and wire closure of the sternal bones (Fig. 57-1B), or costal homograft and mesh repair are but a few of the techniques for repair in the neonate (2). Autologous repair can usually be performed in infants and adolescents (3). Recurrences following repair generally involve a nonunion of the two joined halves of the bifid sternum. Reoperation must include removal of any scar tissue to ensure a bony union. The most severe form of sternal cleft, the total cleft or bifid sternum (Fig. 57-1C), involves the entire sternum, and as a result, is associated with ectopia cordis (4). The most serious array of anomalies associated with a sternal cleft is the Pentalogy of Cantrell. This association consists of a cleft of the lower sternum, epigastric omphalocele, anterior diaphragm defect, and pericardial and intracardiac defects (5).
Combined Musculature and Bony Defects
The muscles of the chest wall (pectoralis major and minor) are also derived from the splitting of dermatomyotomes in the thoracic region shortly after the fifth week postfertilization and is completed by 7 weeks. The classic deformity of muscles and bones of the chest wall is called Poland syndrome (6,7,8). Poland syndrome patients usually present with a chest wall defect lateral to the sternum. The anterior ribs and pectoralis major muscle may be absent. The pectoralis minor muscle is usually normal.
Rib and Car tilage Anomalies
Absent ribs are significant for overall chest wall development and exposure of the underlying pleura and lungs. Absent ribs can be observed through the formative years provided there is no compromise to the underlying structures. Frequently, axial growth makes these problems resolve. If chest wall reconstruction is necessary, this is possible with autologous perichondrium and bone grafts as well as mesh repair.
PECTUS EXCAVATUM
The etiology of pectus excavatum is unknown. It occurs in approximately 1:400 live births. There is nearly a 10% incidence in children with elastic tissue abnormalities (e.g., Marfan syndrome and Ehlers Danlos syndrome (9,10). A familial incidence is seen, and male predominance approaches a ratio of 5:1 (11). The excavatum deformity is a result of abnormal growth, lengthening, and rotation of the costal cartilages, all leading to a depression of the sternum. Differential cartilaginous growth can result in significant asymmetry of the chest wall with a classic rotation of the sternum down to the right and the appearance of the left side of the sternum elevated compared with the right. Analysis of the cartilage from children with pectus excavatum shows differences in biomechanical and histochemical properties, but not in morphology compared with children with a normal postmortem exam. The cartilage derived from pectus excavatum exhibits decreased tension, compression, and flexure. Moreover, type II collagen patterns in the deep zones of
cartilage is disturbed, yet proteoglycan, the other component of cartilage matrix, is unaffected (12). The literature is replete with descriptive analyses of pectus excavatum, but molecular observations may eventually point to a mechanism.
cartilage is disturbed, yet proteoglycan, the other component of cartilage matrix, is unaffected (12). The literature is replete with descriptive analyses of pectus excavatum, but molecular observations may eventually point to a mechanism.
Perhaps the most controversial area for this anomaly centers around the magnitude and significance of its physiologic sequelae. The notion that pectus excavatum creates cardiopulmonary compromise and demands reconstruction has eluded proof from prospective randomized trials. Thus, the indications for repair and for what reason are still subject to opinion based on primarily nonrandomized class II (retrospective) data. Historically, physiologic testing for children with pectus excavatum has included pulmonary function studies and heart function by evaluation of electrocardiogram (ECG), Holter monitor, and echocardiography. Static pulmonary function tests are not helpful for making any recommendations. In children and teenagers, these “at rest” studies usually show little to no abnormalities (13). Exercise pulmonary function studies have shown a restrictive pattern in the 10- to 12-year-old and teenager characterized by statistically significant lower lung volumes (14,15). Reduced maximal exercise capacity secondary to reduced cardiovascular performance (threshold for lactate accumulation) rather than ventilatory limitations (total lung capacity and residual volume) has been reported in a cohort of patients ages 13 to 50 years (16). Evaluation of cardiac function points to diminished right ventricular filling, but this seemingly important observation has eluded class I randomized evaluation (17). Moreover, overall cardiac function during exercise seems to not be affected (18). The psychological and psychosomatic aspects associated with pectus excavatum influence every aspect of life for children and teenagers. Embarrassment reactions, social anxiety, feelings of stigma, limited capacity for work, orientation toward failure, reduced tolerance to frustration, and marked depressive reactions are characteristic of children older than age 11 (19). These are important issues when dealing with the indications for repair and collectively demand that all health care providers be advocates for treatment of this congenital anomaly.
Clinical Diagnosis and Management
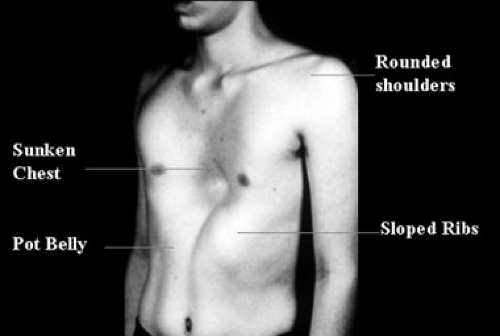
Clinically significant pectus excavatum can be seen in the newborn, but the most common time for initial appearance is in the 2- to 3-year-old and a second group in early childhood. Least common is the presentation of a pectus excavatum during the teenage pubertal growth spurt. Newborns with a pectus excavatum typically have paradoxical movement of the chest wall with respirations. During inspiration, the sternum retracts into a more posterior position and the chest wall expands laterally. There is no role for the repair of a pectus excavatum in the newborn period. Even toddlers should be followed yearly with the notion that 50% of these anomalies have the potential to spontaneously resolve. The typical patient is a school-age child seeking consultation for a visible anatomic deformity of the chest wall. On rare occasions, pain is a presenting symptom, but this is more likely to accompany a child who becomes short of breath during exercise. These children generally do not have a past medical history of reactive airway disease, but may carry a diagnosis of “exercise-induced asthma.” They also have no symptoms at rest. Older children, teenagers, and young adults will undoubtedly present with a cosmetic complaint. Associated findings include rounded shoulders, sloped ribs, protuberant abdomen, and the classic symmetric funnel chest deformity, typically involving the lower to mid-sternum (Fig. 57-2). With moderate to severe degrees of sternal depression, the heart is typically displaced into the left hemithorax such that the point of maximal intensity may be at the anterior axillary line.
As axial growth spurts proceed, the single most important observation for an irreversible excavatum deformity is the presence of asymmetry or rotation of the sternum secondary to differential growth of the cartilages. Typically, this involves rotation of the sternum to the right such that the left side of the sternum is higher than the right. Anatomic measurements can help document the severity of the defect. These observations can be accomplished with a computed tomography (CT) scan of the chest or by using a handheld caliper. The CT scan is the most reliable study for documenting displacement of the heart and lung volumes (20). An index of severity [Pectus index (PI)] can be obtained by measuring the ratio of the distance between the sternum and vertebral bodies and the overall transverse diameter of the chest at the deepest portion of the defect. In normal children this ratio is never greater than 2.5. In children with the pectus excavatum deformity, the index ranges from 3 to 6 (Fig. 57-3). The simpler caliper
measurement of 2.5 cm or greater correlates with a PI greater than 3.0.
measurement of 2.5 cm or greater correlates with a PI greater than 3.0.
Candidates for repair should be evaluated for severity of the defect as outlined previously and undergo stress pulmonary function studies. If history or pulmonary stress function warrants cardiac evaluation, an echocardiogram should be added to the evaluation. In addition, a screening exam for connective tissue disorder may be indicated in some children, but especially important in a patient with a positive family history. The presence of double joints, hyperflexibility, repeated prescription changes for eyes, a history of joint dislocation, or history of mitral valve prolapse all warrant connective tissue screening.
In patients with defects in the moderate to severe category (PI > 3.0), guidelines for operative correction include chest pain, dyspnea on exertion, exercise intolerance or fatiguability, cosmetic concerns, exercise-induced asthma, cardiac function abnormalities, significant psychological distress, and the possibility of median sternotomy for future cardiac surgery in children with Marfan syndrome. Simultaneous repair of pectus excavatum and congenital heart defects can be performed without serious complications (21). The timing of repair is also subject to much debate. Since the mid-1990s, it has become clear that this is not a congenital anomaly that should be corrected younger than the age of 7 because of the higher incidence of potential damage to the growth centers of the cartilage resulting in acquired Jeune syndrome (22,23). Moreover, toddlers can be followed expectantly with yearly exams and no sequelae. Most repairs are well tolerated in older children and adolescents (ages 8 to 12). No repairs should be performed during a growth spurt, regardless of age. If possible, one to two growth spurts should follow the repair. However, this is highly dependent on what age the consult occurs. Symptomatic adults have shown significant improvement following repair of the excavatum deformity (24,25). In one cohort of adults with pectus excavatum (16 to 68 years old), relief of medical symptoms approached 90%, indicating that even in symptomatic adults repair can be performed safely with excellent long-term results (12 ± 7 years) (26).
Operative Techniques
For nearly 60 years, the Ravitch procedure with minor modifications, such as stainless steel bar supports for the sternum, had been the benchmark procedure for pectus excavatum (27). In 1998, Nuss introduced a minimally invasive technique for pectus excavatum that involves splinting the sternum with a steel bar (28). The Nuss procedure achieves correction without the requirement of subperichondrial cartilage excision. This minimal invasive technique for repair of pectus excavatum has now been accepted as an alternative method of repair around the world. Evidence-based rationale for the use of this repair is finding its way into the literature, with many surgeons offering this repair as their procedure of choice. Unfortunately, no study to date has answered the question of a defined pathophysiologic “indication” for the operation. Because opinion and guidelines continue to be published, “standards” for surgical indications may well elude pediatric surgeons for a long time as surgeons are uncomfortable simply observing a patient with a severe defect with symptoms.
THE MODIFIED RAVITCH PROCEDURE (FIG. 57-4A–C, TABLE 57-1)
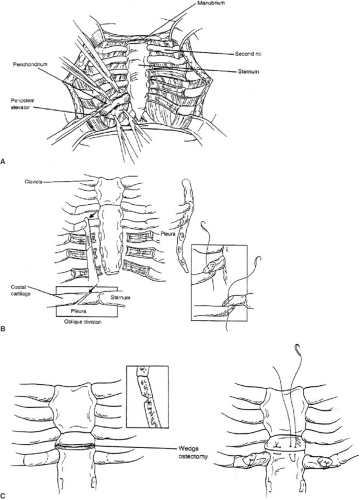
The principal concept of the Ravitch operation involves resection of all abnormal costal cartilages (29). The sternum is anatomically normal, but displaced by the overgrowth in length of the cartilages; therefore, it only needs to be fractured on its anterior table to restore a normal position, once it has been freed from the costal cartilages. A
transverse inframammary skin incision through the deepest portion of the sternal defect is the most appealing from a cosmetic perspective. Superiorly and inferiorly based subcutaneous skin flaps, followed by pectoralis muscles flaps are created exposing the costal cartilages in their entire length bilaterally. Typically, this exposure involves cartilages 4 to 7 or, in more severe cases, can involve the second or third cartilage bilaterally. A minimum of four cartilages should be excised bilaterally. A normal cartilage is always exposed cranial to the uppermost abnormal cartilage. The deformed costal cartilages are resected in a subperichondrial fashion from their union with rib to their junction with the sternum. The perichondrium is incised anteriorly along each cartilage and cephalad and caudad flaps created to expose the defective cartilage. Meticulous care is needed during the posterior portion of the dissection to avoid entering the pleural space. The perichondrium must be preserved in its entirety because it is from this tissue that new cartilage and subsequent bone is generated. Moreover, devascularization of the perichondrium may be a major cause for an acquired thoracic chondrodystrophy years later, following pectus reconstruction. A substernal plane is created by exposing and elevating the xiphoid and sweeping the pleura off the underside of the sternum. Meticulous care is taken to preserve both the pleura and the pericardium. The perichondrial bundles are then detached from the sternum to the uppermost resected cartilage. The internal mammary artery is swept laterally and should remain with the detached perichondrial bundles. An anterior triangular-shaped sternal osteotomy is made just above the resected cartilages (usually between cartilages 2 and 3). An oblique osteotomy will resolve the rotation of the sternum to a more anterior and neutral midline. A second, more caudal, anterior osteotomy is sometimes indicated. To further support the sternum, tripod supports can be created using the lowest of the intact costal cartilages (usually the third). Oblique division of this cartilage from medial to lateral facilitates positioning the medial portion atop the lateral, thereby supporting the sternum in its anterior position. The periosteum of the sternum is sutured to further secure the sternum in its neutral position. For children older than the age of 10 or those with elastic cartilage abnormalities (Marfan syndrome, Ehlers-Danlos syndrome), additional sternal support is recommended with a substernal stainless steel bar beneath the distal third of the sternum and secured to ribs (Fig. 57-5). Lateral support for the strut is achieved by securing it to the medial ends of an appropriate rib. The bar is anchored to the ribs using interrupted sutures. Detached intercostal bundles do not need to be sutured to the sternum. However, if the distance between the sternum and resected bundle is no more than 2 to 3 cm, the defect may be closed with approximation of these tissues. We typically reapproximate the last detached intercostal bundle to the lateral sternal border to ensure proper alignment. A chest tube is placed in the retrosternal space and a subcutaneous drain is placed over the muscle to prevent fluid collections; these are typically removed on postop days 2 and 3. Postoperative pain is moderate and adequately managed with intravenous patient-controlled analgesia. Once oral analgesia is established and drains removed, discharge is feasible. Children are usually discharged on hospital day 3 or 4. Early complications include pneumothorax, hemothorax, subcutaneous fluid collections, wound infections, urinary retention, and atelectasis. Urinary retention is common in the teenager and older patient. Therefore, a bladder catheter is recommended, but not mandatory for postop days 1 and 2. Use of postop incentive spirometer is suggested to avoid atelectasis. Taken together, these are all rare complications
and occur in less than 5% of patients. Their incidence is slightly higher in the adult population because of the magnitude of the dissection and the fact that the cartilage is now bone. The requirement for transfusion is extremely rare. Patients are released to home with limited activities. Contact sports are avoided for 6 to 8 weeks. This is typically enough time for regrowth of the cartilages and fixation of the sternum into its new neutral position, regardless of the patient’s age. The retrosternal strut remains in place for at least 6 months and can then be removed in the outpatient setting. Long-term complications include migration of the bar and recurrence, which may occur in up to 5% to 12% of patients in some series (30,31,32,33,34). The recurrence is slightly higher for the younger, school-age child probably because of the number of subsequent growth spurts. Recurrent pectus excavatum following the modified Ravitch technique can be successfully repaired using the same technique. Alternatively, use of other techniques such as autologous bone graft, cartilage grafts, muscular reconstructions, or the Nuss procedure have been used with success (35). Recurrence seems to be more prevalent if the operation was done at a very early age (less than 5 to 7 years old) and in older teenagers (15 to 17 years old).
transverse inframammary skin incision through the deepest portion of the sternal defect is the most appealing from a cosmetic perspective. Superiorly and inferiorly based subcutaneous skin flaps, followed by pectoralis muscles flaps are created exposing the costal cartilages in their entire length bilaterally. Typically, this exposure involves cartilages 4 to 7 or, in more severe cases, can involve the second or third cartilage bilaterally. A minimum of four cartilages should be excised bilaterally. A normal cartilage is always exposed cranial to the uppermost abnormal cartilage. The deformed costal cartilages are resected in a subperichondrial fashion from their union with rib to their junction with the sternum. The perichondrium is incised anteriorly along each cartilage and cephalad and caudad flaps created to expose the defective cartilage. Meticulous care is needed during the posterior portion of the dissection to avoid entering the pleural space. The perichondrium must be preserved in its entirety because it is from this tissue that new cartilage and subsequent bone is generated. Moreover, devascularization of the perichondrium may be a major cause for an acquired thoracic chondrodystrophy years later, following pectus reconstruction. A substernal plane is created by exposing and elevating the xiphoid and sweeping the pleura off the underside of the sternum. Meticulous care is taken to preserve both the pleura and the pericardium. The perichondrial bundles are then detached from the sternum to the uppermost resected cartilage. The internal mammary artery is swept laterally and should remain with the detached perichondrial bundles. An anterior triangular-shaped sternal osteotomy is made just above the resected cartilages (usually between cartilages 2 and 3). An oblique osteotomy will resolve the rotation of the sternum to a more anterior and neutral midline. A second, more caudal, anterior osteotomy is sometimes indicated. To further support the sternum, tripod supports can be created using the lowest of the intact costal cartilages (usually the third). Oblique division of this cartilage from medial to lateral facilitates positioning the medial portion atop the lateral, thereby supporting the sternum in its anterior position. The periosteum of the sternum is sutured to further secure the sternum in its neutral position. For children older than the age of 10 or those with elastic cartilage abnormalities (Marfan syndrome, Ehlers-Danlos syndrome), additional sternal support is recommended with a substernal stainless steel bar beneath the distal third of the sternum and secured to ribs (Fig. 57-5). Lateral support for the strut is achieved by securing it to the medial ends of an appropriate rib. The bar is anchored to the ribs using interrupted sutures. Detached intercostal bundles do not need to be sutured to the sternum. However, if the distance between the sternum and resected bundle is no more than 2 to 3 cm, the defect may be closed with approximation of these tissues. We typically reapproximate the last detached intercostal bundle to the lateral sternal border to ensure proper alignment. A chest tube is placed in the retrosternal space and a subcutaneous drain is placed over the muscle to prevent fluid collections; these are typically removed on postop days 2 and 3. Postoperative pain is moderate and adequately managed with intravenous patient-controlled analgesia. Once oral analgesia is established and drains removed, discharge is feasible. Children are usually discharged on hospital day 3 or 4. Early complications include pneumothorax, hemothorax, subcutaneous fluid collections, wound infections, urinary retention, and atelectasis. Urinary retention is common in the teenager and older patient. Therefore, a bladder catheter is recommended, but not mandatory for postop days 1 and 2. Use of postop incentive spirometer is suggested to avoid atelectasis. Taken together, these are all rare complications
and occur in less than 5% of patients. Their incidence is slightly higher in the adult population because of the magnitude of the dissection and the fact that the cartilage is now bone. The requirement for transfusion is extremely rare. Patients are released to home with limited activities. Contact sports are avoided for 6 to 8 weeks. This is typically enough time for regrowth of the cartilages and fixation of the sternum into its new neutral position, regardless of the patient’s age. The retrosternal strut remains in place for at least 6 months and can then be removed in the outpatient setting. Long-term complications include migration of the bar and recurrence, which may occur in up to 5% to 12% of patients in some series (30,31,32,33,34). The recurrence is slightly higher for the younger, school-age child probably because of the number of subsequent growth spurts. Recurrent pectus excavatum following the modified Ravitch technique can be successfully repaired using the same technique. Alternatively, use of other techniques such as autologous bone graft, cartilage grafts, muscular reconstructions, or the Nuss procedure have been used with success (35). Recurrence seems to be more prevalent if the operation was done at a very early age (less than 5 to 7 years old) and in older teenagers (15 to 17 years old).
TABLE 57-1 Operative Procedures for Repair of Pectus Excavatum. | |
|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree