Subglottic Airway
Richard G. Azizkhan
Cincinnati Children’s Hospital Medical Center, University of Cincinnati School of Medicine, Cincinnati, Ohio 45229.
Many different airway disorders can cause airway obstruction and respiratory distress in children, and determining a precise diagnosis requires an understanding of each disorder. Due to distinctive anatomic features of the pediatric airway, symptoms of respiratory distress may rapidly progress and become life threatening. It is thus incumbent for physicians to quickly determine the problem and manage it appropriately.
Management strategy generally depends on the degree of respiratory insufficiency and information obtained from a thorough case history. Less severe respiratory insufficiency often manifests in subtle symptoms, such as irritability, restlessness, tachycardia, and feeding difficulties. Cyanosis, severe suprasternal and intercostal retractions, tachypnea, and lethargy indicate more severe respiratory compromise. Stridor, a harsh respiratory sound produced by turbulent airflow, is the most important physical sign in cases of upper airway obstruction. It can be present either in the inspiratory or expiratory phase of the respiration, or in both (1,2). Its characteristics, as well as its relationship to the respiratory cycle, may help in establishing a definitive diagnosis and in setting priorities for diagnostic evaluation (2). Reviewing the history of the child’s symptoms may provide valuable clues to underlying etiology. Close attention should be paid to circumstances that may have triggered the onset of respiratory compromise, to the duration of symptoms, and to their progression over time. Questioning parents about a history of dysphagia or feeding problems, the nature of their child’s cry, and the possibility of foreign-body aspiration can also yield important information. In addition, any previous history of endotracheal intubation, trauma, or cardiopulmonary abnormalities should be carefully reviewed.
In any patient with airway compromise, control of the airway is imperative and is the primary clinical concern. Once airway and ventilatory control has been obtained, a thorough evaluation should be initiated to delineate the structural lesions or functional abnormalities of the subglottic airway. This chapter focuses on these disorders, as well as the diagnostic and therapeutic techniques that are crucial to their successful management. To set the stage for this discussion, an overview of the embryogenesis and relevant anatomy of the pediatric airway is presented.
TRACHEOBRONCHIAL EMBRYOLOGY
The laryngotracheal groove arises from the ventral surface of the foregut during the third and fourth weeks of gestation (3). Cells lining the celomic cavity also divide during this time, becoming a proliferating primitive mesenchyme and eventually pulmonary muscle, cartilage, and connective tissue. During the fifth week of gestation, caudal progression of the embryonic trachea is followed by bifurcation and the appearance of lung buds. During the sixth week, the lobar bronchi lengthen and abut the esophagus. Asymmetric buds (the left bud being shorter and more nearly horizontal than the right) rapidly divide into lobar bronchi and tertiary bronchi by the seventh week. At this stage, pseudostratified epithelium lines the larger airways. Differentiation and branching of the epithelial bronchial tree depends on the presence of the mediastinal mesenchyme (3,4). The formation of new smaller bronchi continues through the sixteenth week. Bronchial subdivisions continue until the seventeenth order is established between the sixth and seventh month. At that time, alveolar differentiation occurs at the site of the distal terminal bronchi and continues until well after birth.
The initial vascular supply of the differentiating bronchial buds develops from the splanchnic plexus that originates from the dorsal aorta and drains into the plexus of cardinal veins. The bronchial arterial blood supply develops from gradual interconnection with the sixth aortic arch, and the bronchial vasculature is left as a remnant of the embryonic vascular system. Tracheal bifurcation is
initially high in the cervical region, but descends to the level of the first thoracic vertebra by 8 weeks of gestation and to the fourth thoracic vertebra at birth. Cartilage appears in the trachea in approximately 10 weeks and in the segmental bronchi at 16 weeks (4). After this time, fetal lung development primarily consists of the successive generation of terminal airways and alveoli.
initially high in the cervical region, but descends to the level of the first thoracic vertebra by 8 weeks of gestation and to the fourth thoracic vertebra at birth. Cartilage appears in the trachea in approximately 10 weeks and in the segmental bronchi at 16 weeks (4). After this time, fetal lung development primarily consists of the successive generation of terminal airways and alveoli.
Congenital anomalies of the respiratory system are relatively uncommon and can occur along the entire tracheobronchopulmonary axis. Associated extrapulmonary anomalies are frequently present and may be part of a syndrome or association. Approximately 45% of children with congenital airway obstruction have associated congenital anomalies (1,2,5), some of which have a significant impact on prognosis. Although the cause of such anomalies is usually unknown, they are believed to result from causal mechanisms that differ in time, mode of action, and the embryonic region affected. As such, they are failures of normal growth and differentiation of different parts of the embryonic respiratory system. More specifically, defective mesodermal development in early embryogenesis may be responsible for the VACTERL association. Primary abnormalities of the developing lung bud itself may lead to a variety of abnormalities, including congenital lobar emphysema, bronchogenic cysts, sequestrations, and cystic adenomatoid malformations, which are discussed elsewhere. Gestational teratogens may have selected effects on specific organ development. Experimental evidence suggests that vitamin A may play an important role in the development of the airway and lungs. In animal embryos, its deficiency causes a keratizing metaplasia of the tracheobronchial tree and pulmonary agenesis (6). Retinoic acid appears to be important for embryonic cell differentiation in many organ systems and has been studied intensely (3). Investigators have described a relationship between the maternal use of valproic acid and both tracheomalacia and laryngeal hypoplasia in offspring (7). Also, a constellation of abnormalities, including severe congenital tracheal stenosis, has been reported in infants of diabetic mothers (8).
PEDIATRIC AIRWAY ANATOMY
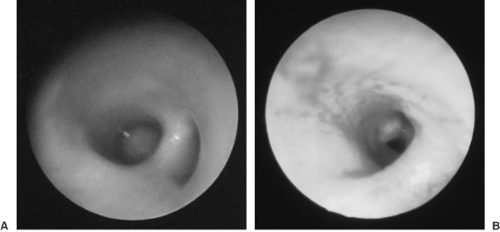
Several anatomic features of the airway in infants differ from those of older children or adults (5,9) and are important to keep in mind because of their impact on symptom progression, diagnosis, and disease management. The caliber of the airway is small and can be readily obstructed by secretions or mucosal swelling. The larynx is more cephalad and anterior, making visualization more difficult, especially for the inexperienced clinician. The cricoid, which is the only completely circumferential laryngeal cartilage, is the narrowest portion of the child’s upper airway. Last, the length of the trachea is very short (approximately 4 to 5 cm), thus increasing the risk of unplanned extubation or right mainstem intubation during flexion or extension of the infant’s head and neck.
Because of this unique subglottic anatomy, care must be taken to minimize trauma to this region during endotracheal intubation and airway procedures. Tightly fitting endotracheal tubes may cause mucosal or submucosal injury, thus resulting in stenosis at the level of the cricoid cartilage. A 50% reduction in cross-sectional area results for every millimeter of airway edema or narrowing of the lumen of an infant’s airway. The correct fit of the child’s uncuffed endotracheal tube can be estimated as follows (10):
4 + (years of age/4) = tube size (mm)
When positioning the endotracheal tube, there must be an air leak at less than 18 to 25 cm of water pressure to ensure an appropriate fit (10).
PATHOPHYSIOLOGY
Mapping the respiratory tract into three distinct regions helps identify possible pathologic correlates of stridor (10). The first area comprises a supraglottic and supralaryngeal region, which includes the pharynx; the second is an extrathoracic tracheal region, which includes the glottis and the subglottis; and the third is made up of an intrathoracic tracheal region, which includes primary and secondary bronchi. In certain regions, stridor occurs more frequently during inspiration, whereas in other regions, it occurs more frequently during expiration. These patterns of stridor are of utmost importance in that they indicate possible etiologies that warrant further investigation or signal imminent emergency. In the first region, stridor generally occurs during inspiration. A patient showing this pattern should undergo careful investigation for upper airway lesions (e.g., choanal atresia), expanding lesions in the tongue (e.g., a dermoid cyst or an internal thyroglossal duct cyst), or lack of structural airway support from mandibular hypoplasia, as seen in Pierre-Robin sequence or from macroglossia, as seen in Beckwith-Wiedeman syndrome. In the second region, stridor is heard during both inspiration and expiration, and is referred to as biphasic. When this pattern is heard, the glottic and subglottic lumina have reached a critically small size and tremendous effort is required to move air through a pinpoint opening. Biphasic stridor often signals impending respiratory collapse and is thus a medical emergency likely to require intubation or tracheotomy. In the intrathoracic bronchial region, the relative positive pressures of expiratory forces within the chest wall narrow the bronchial lumen in
normal children. As air moves during expiration, the Venturi principle adds a constricting force. In the presence of a bronchial foreign body or a lesion, these forces act jointly to close the airway lumen. The expiratory phase sound can be heard as either stridor or wheezing.
normal children. As air moves during expiration, the Venturi principle adds a constricting force. In the presence of a bronchial foreign body or a lesion, these forces act jointly to close the airway lumen. The expiratory phase sound can be heard as either stridor or wheezing.
AIRWAY ENDOSCOPY
Airway endoscopy, also referred to as laryngotracheobronchoscopy, is the instrument-aided visual examination of the airway. It can reveal airway structure and dynamics as well as airway contents, and also provide access to foreign bodies, secretions, and washings from the lower airways. As such, it has increasingly been used in infants and children for both diagnostic and therapeutic purposes (11,12,13). For a child with signs of respiratory distress, chest wall retractions, severe stridor, or tripod posture with drooling, airway endoscopy is urgent and imperative (10). Early endoscopic intervention in the setting of worsening stridor may avoid more extreme measures such as endotracheal intubation or a tracheotomy. Both of these procedures may interfere with establishing an accurate diagnosis. In cases where some degree of airway obstruction coexists with feeding difficulties and insufficient weight gain, airway endoscopy is warranted to determine the underlying cause. These circumstances are more likely to be found in chronic conditions such as laryngomalacia and bronchotracheomalacia. When diagnostic imaging techniques suggest an abnormality such as a vascular ring, endoscopy may be required to confirm the diagnosis. Because a significant percentage of children with stridor have more than one airway lesion, the entire upper and lower airway should be examined unless there are contraindications such as critical tracheal stenosis (11).
For airway endoscopy to be carried out safely and effectively, cooperation and collaboration between the surgeon and the anesthesiologist are absolutely essential, and an overall strategy must be discussed and agreed upon. Optimally, the attending pulmonologist should be included in the operative team, particularly when the patient has a history of pulmonary dysfunction. Also, essential resources should be available in the event of the need for urgent airway access by cannula or a tracheotomy.
RIGID AND FLEXIBLE BRONCHOSCOPY
Although rigid and flexible bronchoscopy offer different advantages and disadvantages, they are best viewed as complementary techniques to assess airway anatomy and function. They are often used concurrently, and of utmost importance, they both require gentle technique. They also both require an appreciation of the unique anatomy of infants and children, and the potential risks involved in attempting to visualize airway structures.
Rigid Bronchoscopy
Rigid bronchoscopy requires the administration of a general anesthetic to prevent pain and potentially dangerous movement that might cause tracheal or laryngeal damage. Because the rigid bronchoscope must be passed through the mouth, proper placement requires some degree of neck hyperextension, thus presenting a significant risk to children with certain physical disabilities such as Down syndrome and Arnold Chiari malformation. This bronchoscope does, however, offer superior visualization and greater suction capabilities. In addition, it allows for the use of a greater variety of instruments in the airways. The removal of foreign bodies is usually performed more safely using rigid bronchoscopy, although flexible bronchoscopy does have a role in the diagnosis of possible foreign-body aspiration (14). Rigid bronchoscopy can be useful in the diagnosis and treatment of massive hemoptysis, in dilatation of tracheal or bronchial stenosis, and in airway stent placement (10). It is also usually advantageous to use a rigid bronchoscope in the evaluation of children with suspected laryngoesophageal clefts, bilateral vocal cord paralysis, or H-type tracheoesophageal fistula. Confirming the importance of rigid bronchoscopy in the diagnostic process, a large-scale, long-term study conducted by Wiseman and colleagues found that it contributed to the final diagnosis in approximately 88% of patients. Further, there was no mortality and a morbidity rate of only 3.5% (15).
Rigid (open-tube) bronchoscopes range from approximately 2.5 to 8.5 mm in diameter to 20 to 50 cm in length. Due to their size, they can function as an endotracheal tube, allowing patients to ventilate. The airways distal to the tip of the bronchoscope are illuminated either with a prism inserted partially into the lumen or by a glass rod telescopic lens (Fig. 59-1). The optical characteristics of the rigid bronchoscope, when used with the glass rod telescope, are excellent and unequaled by any other bronchoscopic device (Fig. 59-2). When the telescope is not used, visualization through the bronchoscope is more difficult. This open-tube endoscopic configuration is often necessary to manipulate instruments (11).
The large lumen of the rigid bronchoscope allows for easy passage of instruments such as forceps, suction catheters, snares, and retrieval baskets into the airways. Because the glass rod telescope takes up a large part of the lumen, special instruments have been designed for use with this device. These include the optical forceps, which operate in conjunction with the telescope, and the ultrathin forceps, which can be passed alongside the telescope. Other endoscopic equipment, such as suction catheters, balloon-tipped catheters, and laser fibers, can also be passed through or around the bronchoscope (11).
Because glass rod telescopes significantly reduce the functional lumen of the rigid endoscope and increase airway resistance, ventilation is not necessarily assured.
This is particularly significant in infants because the most commonly used bronchoscope has an internal diameter of 3.5 mm and a corresponding telescope with a diameter of 2.5 mm. Therefore, it is essential that the patient’s hemoglobin-oxygen saturation and ventilation be carefully monitored throughout the procedure. It is also important to evaluate airway dynamics, which may be altered by sedation or anesthesia, and to examine the airway with the patient spontaneously breathing. This is done because dynamic airway collapse can easily be missed during positive-pressure ventilation. The bronchoscope itself can also alter dynamics, either by stenting the airway or
by changing airway resistance and resultant pressure relationships (11).
This is particularly significant in infants because the most commonly used bronchoscope has an internal diameter of 3.5 mm and a corresponding telescope with a diameter of 2.5 mm. Therefore, it is essential that the patient’s hemoglobin-oxygen saturation and ventilation be carefully monitored throughout the procedure. It is also important to evaluate airway dynamics, which may be altered by sedation or anesthesia, and to examine the airway with the patient spontaneously breathing. This is done because dynamic airway collapse can easily be missed during positive-pressure ventilation. The bronchoscope itself can also alter dynamics, either by stenting the airway or
by changing airway resistance and resultant pressure relationships (11).
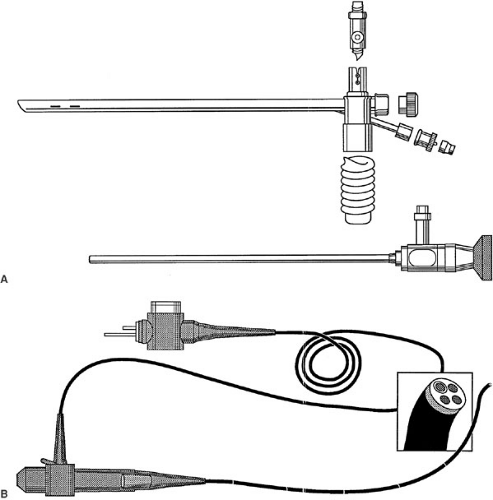 FIGURE 59-1. (A) A Storz pediatric ventilating bronchoscope (top) and a Hopkins rod lens telescope (bottom). (B) Flexible pediatric bronchoscope. |
Flexible Bronchoscopy
Although flexible fiberoptic bronchoscopy can be performed using a topical anesthetic and intravenous sedation, it is often performed under general anesthesia in infants and young children. The scope can be inserted either orally or nasally and can be passed through endotracheal or tracheostomy tubes of appropriate size. Flexible bronchoscopy has also been successfully performed through a laryngeal mask. Whereas the patient undergoing rigid bronchoscopy can use the endoscope for breathing, the patient undergoing flexible bronchoscopy must breathe around the instrument. This endoscopic approach is advantageous in maintaining difficult airways due to poor or limited neck mobility and cervicofacial trauma. Also important, flexible bronchoscopes are able to penetrate further into the bronchial tree with greater peripheral range. They can thus more easily detect distal foreign bodies that would not be seen with a rigid scope (11,12,13,16).
New pediatric flexible bronchoscopes have an outer diameter ranging from 2.8 to 3.7 mm and a 1.2 mm suction channel. They are distinguished by the ability to flex the distal end to as much as 180 degrees (Fig. 59-1B). These bronchoscopes can be used in infants weighing as little as 500 g, and most term infants can breathe around them spontaneously for short periods. The image produced by a flexible bronchoscope is composed of several thousand points of light, each representing the color and light intensity transmitted by a single glass fiber. Although the resolution of this image is lower than that with the glass rod telescope, the perceived image quality is quite good. This can be attributed to the fact that the surgeon’s eye rapidly compensates for the lower resolution (11).
The standard flexible bronchoscope is diagnostically useful in the assessment of atelectasis, and can be used to obtain selected lobar or segmental bronchial washings for cytologic and microbiologic evaluation. Occasionally, lavage with mucolytic agents may be necessary, and rarely, a rigid bronchoscope with forceps extraction must be used in patients who have discrete mucous plugs (11). If the atelectasis is due to an impacted foreign body or a tissue mass, rigid bronchoscopy is needed.
The development of lasers that can be transmitted through fiberoptic light cables has led to the increasing use of laser bronchoscopy in the treatment of some intraluminal sublglottic airway lesions (17). Argon, neodymium-yttrium aluminum garnet (Nd:YAG), and potassium titanyl phosphate (KTP) are the three most common lasers used with this method of delivery, and all these laser systems can be deployed through either rigid or flexible endoscopes (Fig. 59-3). The use of small (300 to 600 μm) quartz fiberoptic laser cables allows clinicians to treat even distal bronchial lesions in small premature infants. The KTP and Argon lasers are most safely and effectively used in the noncontact mode, creating lesions 0.5 mm in diameter. These small spot sizes provide a significant advantage over the Nd:YAG laser, which requires a contact probe and direct contact with the tissues. The thermal effect (i.e., depth of penetration and spot size) of the Nd:YAG laser is more difficult to control and thus not as safe in children (18). Although both the Argon and KTP lasers are particularly useful for removing tracheal or endobronchial granulomas after repair of tracheal stenosis, the KTP laser is currently believed to be the most precise and effective in vaporizing lesions, while causing less destruction to overlying mucosa.
Ultrathin flexible bronchoscopes (1.8 to 2.2 mm in diameter) offer a number of advantages for examination of the lower airway of infants and small children. They
are particularly useful in the critical care setting, where bedside endoscopy can be performed through small endotracheal tubes while maintaining effective ventilation. They are also extremely useful in evaluating the position of endotracheal tubes, examining the dynamics of the posterior wall of the trachea in relation to tracheostomy tubes, and for retrograde laryngoscopy in infants with tracheostomies. Flexible bronchoscopes can be used to direct placement of balloon catheters for bronchial dilatation, in positioning airway stents and fiberoptic laser fibers, and in examining the peripheral airway. A drawback of the ultrathin scope is that it is not equipped with a suction channel. Blood or secretions can thus easily obscure visualization.
are particularly useful in the critical care setting, where bedside endoscopy can be performed through small endotracheal tubes while maintaining effective ventilation. They are also extremely useful in evaluating the position of endotracheal tubes, examining the dynamics of the posterior wall of the trachea in relation to tracheostomy tubes, and for retrograde laryngoscopy in infants with tracheostomies. Flexible bronchoscopes can be used to direct placement of balloon catheters for bronchial dilatation, in positioning airway stents and fiberoptic laser fibers, and in examining the peripheral airway. A drawback of the ultrathin scope is that it is not equipped with a suction channel. Blood or secretions can thus easily obscure visualization.
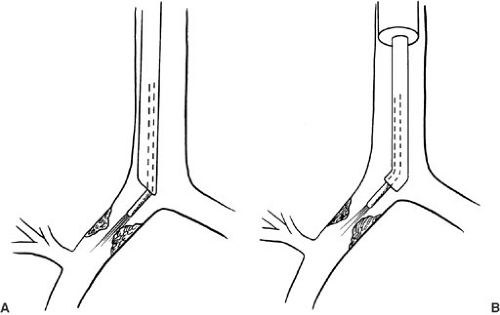
Endoscopic techniques can also be useful during extubation in selected patients. When extubation has been unsuccessful, direct examination of the airway may define the problem (Fig. 59-2). The lower airways are initially examined through the endotracheal tube. The nasopharnyx and larynx are then examined with a flexible bronchoscope that has been passed through an appropriately sized clean endotracheal tube. The indwelling endotracheal tube is withdrawn after the tip of the bronchoscope is positioned just above the glottis. The bronchoscope is withdrawn if the anatomy and function appear favorable after several minutes of observation. If the child has evidence of obstruction or severe respiratory dysfunction, the bronchoscope is advanced into the trachea, and the fresh endotracheal tube is subsequently passed. Because subglottic edema that is significant enough to prevent successful extubation may not become apparent for 5 to 10 minutes after extubation, the airway should be examined in an unhurried manner (11).
Complications of Airway Endoscopy
Although airway endoscopy is a commonly used procedure, it is one that requires skill and experience to perform safely and effectively. The patient must be properly prepared and monitored before, during, and after the procedure. The endoscopist must be able to recognize and deal with any complications that may arise. The most commonly observed complications are due to direct or indirect tissue trauma. These complications include mucosal trauma, hemoptysis or epistaxis, pneumothorax, subglottic edema, tracheal or bronchial perforation, and trauma to the vocal cords. They occur more commonly with rigid bronchoscopy both because of the rigid physical characteristics of the instruments used and because of the types of procedures performed with this approach. Illustrating the latter, mucosal tears or bronchial perforation can occur during foreign-body extraction.
Physiologic complications include hypoxia, hypercarbia, laryngospasm, bronchospasm, and bradycardia or other cardiac arrhythmias. Hypoxia and hypercarbia may occur because a flexible instrument obstructs the airway for a significant period of time or because prolonged manipulation of an instrument through the open channel of a rigid endoscope interrupts positive-pressure ventilation. Cardiac arrhythmias and laryngospasm can result from direct vagal stimulation, often a consequence of insufficient topical anesthesia (11). Inadequate sedation or topical anesthesia can result in mechanical trauma from coughing. If the patient’s stomach is not empty, endoscopy may induce regurgitation or gastroesophageal reflux and may cause aspiration. In addition, passage of a bronchoscope and the associated sedation or anesthesia alter the ability to breathe, placing patients at risk for other complications. Careful monitoring during any endoscopic airway examination is thus essential.
THE SURGICALLY CREATED AIRWAY
The surgeon treating infants and children with tracheobronchial abnormalities must be competent in performing different techniques of establishing a secure airway and with managing the postoperative care that each technique requires. Essential techniques include cricothyroidotomy, tracheotomy (temporary airway), and tracheostomy (long-term airway). The treatment selected is based on the surgeon’s determination of whether securing the airway is emergent or elective and permanent versus temporary.
Cricothyroidotomy
Rarely, when establishing an airway is critical due to an acute airway obstruction episode and orotracheal intubation is not possible, the treatment of choice is a cricothyroidotomy. Immediate percutaneous tracheal access can be obtained with a needle cricothyroidotomy. A 14-gauge intravenous cannula is inserted through the cricothyroid membrane, and high-flow plastic tubing with 100% oxygen is connected to this cannula. Because this technique is a temporizing maneuver, a more secure surgical or orotracheal airway should be obtained as soon as possible. Another option is surgical cricothyroidotomy, which is also an excellent method of rapidly securing a difficult airway in an emergency. Subsequent to this procedure, a larger and more stable endotracheal tube can be inserted through the cricothyroid membrane. Because this approach is, however, known to cause irrevocable injury to the cricoid cartilage and subglottic region when performed in infants and small children, its use should be restricted to older children and teenagers. Conversion to a formal tracheostomy is recommended within a few days in cases likely to require prolonged airway support.
Tracheotomy and Tracheostomy
Whereas airway obstructions secondary to infection were the most common indication for emergency tracheotomy
in the past, this procedure is now commonly performed in infants and children with a wide range of congenital and acquired structural or functional airway abnormalities. Table 59-1 tracheostomy is also often required as an adjunct to airway reconstruction.
in the past, this procedure is now commonly performed in infants and children with a wide range of congenital and acquired structural or functional airway abnormalities. Table 59-1 tracheostomy is also often required as an adjunct to airway reconstruction.
TABLE 59-1 Indications for Tracheotomy in Infants and Children. | ||
|---|---|---|
|
Knowledge of posttracheostomy complications has enabled surgeons to make modifications in tracheostomy technique. Important principles of this technique now include the preservation of tracheal tissue by not excising a window or flap and by not performing the procedure on an infant without a secured airway (19). In preparation for the procedure, the infant or child should be anesthetized in the operating room via endotracheal intubation or through a ventilating rigid bronchoscope. A small roll is placed under the child’s shoulders and the neck is prepared with a surgical antiseptic solution. A transverse cervical skin incision is made 1 to 2 cm above the sternal notch, depending on the size of the child. The cervical fascia is then incised vertically and the thyroid isthmus retracted. Traction sutures made of Prolene are placed on both sides of the trachea, and a vertical tracheotomy is performed through the third and fourth tracheal rings. The surgeon and the anesthesiologist jointly coordinate the slow withdrawal of the endotracheal tube and placement of tracheostomy tube. The traction sutures are taped to the neck for use in guiding the replacement of this tube in the event of dislodgment or unexpected airway obstruction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree