Small Intestine
Martin H. Ulshen
William R. Treem
Division of Pediatric Gastroenterology and Nutrition, Duke University Medical Center, Durham, North Carolina 27710.
Division of Pediatric Gastroenterology and Nutrition, Duke University Medical Center, Durham, North Carolina 27710.
The small intestine is the major digestive and absorptive portion of the gastrointestinal tract. Any pathologic process that disrupts the normal function of the small intestine profoundly affects the normal growth and metabolism of the child. Pediatric surgeons are involved in the care of patients with chronic conditions of the small intestine that preclude normal enteral nutrition. These include conditions resulting in short bowel syndrome and protracted diarrhea in infancy; motility disorders causing pseudoobstruction and bacterial overgrowth; acquired inflammatory immune-mediated conditions, such as Crohn’s disease and graft versus host disease; and acquired conditions believed to be triggered by viral or bacterial infections, such as Henoch-Schönlein purpura and hemolytic uremic syndrome. Parenteral nutrition has provided a means of supporting these patients, while treating their underlying disease and waiting for growth, development, and regeneration of the damaged small intestine. This chapter briefly reviews the ontogeny of small intestine development, the basic gross and microscopic anatomy, the physiology of nutrient digestion and absorption, and normal small intestinal motility. The second portion of the chapter highlights some of the disease processes that disrupt normal function in these areas and lead to the potential need for surgical intervention to provide parenteral or selective enteral nutrition.
DEVELOPMENTAL ANATOMY
The gut lengthens rapidly between 6 and 12 weeks’ gestation. During this time, the small intestine transiently herniates into the umbilical cord. On reentry into the abdominal cavity, the intestine rotates counterclockwise 270 degrees around an axis formed by the superior mesenteric artery. Further positioning of the small intestine continues until 20 weeks’ gestation, when the gastrointestinal tract achieves its final anatomic position. The small intestine continues to lengthen throughout gestation and measures 200 to 300 cm at birth in a full-term neonate (1)..Between 26 and 38 weeks’ gestation, the overall length of the gastrointestinal tract doubles. The small intestine does not achieve its maximum length of 600 to 800 cm until at least 4 years of age. These changes in overall length, the doubling of the intestinal diameter, and the development of the plicae circulares, villi, and microvilli combine to enlarge the absorptive surface area of the small intestine from about 950 cm2 at birth to 7,500 cm2 in the adult (2).
The circular muscles of the small intestine appear at 6 weeks’ gestation, and the longitudinal muscles at 8 weeks’ gestation. Neuroblasts appear at 7 weeks’ gestation, and differentiation into the myenteric and submucosal plexuses occurs at 9 and 13 weeks’ gestation, respectively. Peristalsis first occurs shortly thereafter, but jejunal contractions remain disorganized in the premature infant before 30 weeks’ gestation (3). When radiographic contrast material is injected into the gastrointestinal tract of a fetus in utero before 30 weeks’ gestation, there is little movement of the marker out of the stomach (4). With the appearance of the migrating motor complexes (MMCs) at 32 to 34 weeks’ gestation, duodenal and jejunal contractions become more coordinated, but are still immature. Despite the greater frequency of MMCs in premature infants, transit time through the small intestine can be as long as 9 hours, or twice as long as that in term infants, because of the twofold shorter propagation rate of the MMCs. By 38 weeks’ postconception, the MMCs are fully present, and fasting motor activity is mature (5). Feedings increase the number of duodenal contractions in infants as young as 25 weeks’ postconception; in very premature infants, there is often the coexistence of an active fed pattern with an immature fasting pattern and no MMCs (6). The administration of corticosteroids accelerates maturation of neonatal small intestinal motility, whereas ischemia and central nervous system disease slow maturation.
Villi appear in the duodenum at 8 weeks’ gestation and proliferate aborally, reaching the terminal ileum by 11 weeks’ gestation. The crypt compartment of the small intestine, which is the site of enterocyte proliferation, appears in the duodenum by 10 weeks’ gestation. The villi acquire their tall, fingerlike shape in the proximal intestine by 14 weeks’ gestation, but remain shorter in the distal small intestine, resulting in a fourfold greater absorptive surface area in the jejunum than in the ileum. Microvilli also appear at 8 weeks’ gestation, and by 14 weeks’ gestation, enterocytes are morphologically mature and display a well-organized brush border. The entire process of enterocyte migration from crypt to villus in the newborn infant is slower than that in adults and requires at least 6 days for epithelial renewal.
ONTOGENY OF DIGESTION AND ABSORPTION
Carbohydrates
The proportion of carbohydrate ingested as starch increases progressively during the first year of life. Starch digestion is dependent on pancreatic α-amylase activity in the duodenal fluid, which is reduced during the first 6 months of life. Below this age, salivary amylase may be important in starch digestion. In addition, α-amylase activity is higher in human milk than in the duodenal contents of infants younger than 3 months of age. By 1 year of age, the capacity for starch digestion is comparable to that of an adult.
Despite the mature appearance of the intestinal epithelium by the end of the second trimester, its brush-border membrane is functionally immature. The disaccharidases lactase and sucrase-isomaltase can be detected by 8 weeks’ gestation, but lactase activity remains low until 36 weeks’ gestation (7). Peak lactase activity is found in the full-term newborn and during early postnatal life. Within the first few years of life, lactase activity decreases to adult levels (one-tenth that at birth). In contrast, sucrase-isomaltase activity rises throughout gestation so 70% of adult levels is achieved at 34 weeks’ gestation and adult levels are reached at birth. Glucoamylase (maltase) has been detected at 13 weeks’ gestation, and the presence of this α-glucosidase together with sucrase-isomaltase allows the premature infant to digest glucose polymers more readily than lactose. These enzymes are also important in the final steps of starch digestion. Despite the apparent lactase deficiency, term infants tolerate lactose-containing formulas and human milk owing to the process of carbohydrate salvage through fermentation to short-chain fatty acids by colonic bacteria. Infant formulas designed for preterm infants often contain lower lactose and higher glucose polymer concentrations.
The rate of glucose transport is low in the first trimester and increases as the number of sodium-coupled transport sites increases first in the jejunum and then in the ileum. The maximal glucose transport rate in the jejunum of infants is 20% to 25% that of adults and increases throughout the first year of life (8).
Proteins
Most protein hydrolysis occurs in the proximal gut and is dependent on the presence of enterokinase, pancreatic proteases, and brush-border and cytosolic peptidases. Enterokinase initiates the process of protein digestion by the activation of trypsinogen. Enterokinase activity is detected in the duodenum at 24 weeks’ gestation and increases during the latter part of gestation to 10% of eventual adult levels, which are finally achieved by about 4 years of age. Despite the relative deficiency of enterokinase, the presence of the pancreatic proteases (trypsin, chymotrypsin, and carboxypeptidase B) permits efficient digestion of protein to peptides. Most of the activities of both the brush-border and cytosolic peptidases are present at 8 weeks’ gestation and soon reach levels similar to those found in adults.
Fats
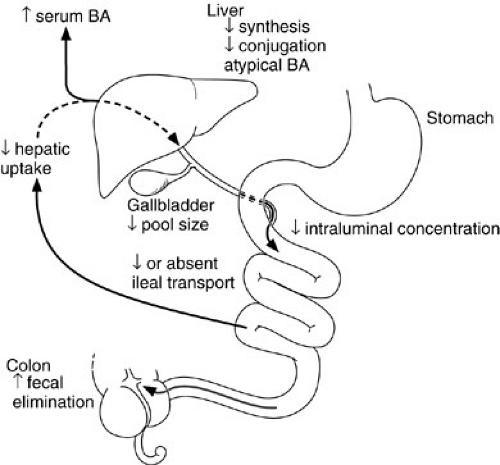
The efficiency of long-chain triglyceride absorption ranges from 70% to 90% in term infants and from 40% to 90% in premature infants. Medium-chain triglycerides are digested and absorbed more efficiently than are long-chain triglycerides because they do not require bile salts for esterification or solubilization in mixed micelles. Medium-chain triglycerides are taken up directly in the portal vein. The multiple factors that contribute to the immaturity of bile acid metabolism and the enterohepatic circulation of bile acids in the newborn are collectively termed physiologic cholestasis (9,10) (Fig. 74-1). These factors include decreased rates of bile acid synthesis, the lack of an active transporter of bile salts in the ileum, and the resultant smaller pool size (11). Although taurine-conjugated bile acids are passively absorbed by the fetal gut, active ileal transport does not begin until after birth. Together, these factors contribute to the relative paucity of bile salts appearing in the infant duodenum; these may not reach the critical micellar concentration necessary for long-chain fat absorption, especially in premature infants. Hormones such as glucocorticoids and thyroxine, growth factors such as epidermal growth factor, and dietary factors including long-chain fatty acids are all believed to regulate and enhance the development of bile acid metabolism and the enterohepatic circulation in the fetus and premature infant (12).
NORMAL ANATOMY AND PHYSIOLOGY
Gross Anatomy
Although a distinct demarcation between jejunum and ileum is not apparent, structural differences are present
that reflect the functional compartmentalization of the small intestine. The thickness of the small bowel wall, the overall luminal diameter, and the prominence of the circular submucosal folds (plicae circulares) are all greatest in the proximal jejunum and decrease with progression through the ileum. Absorptive surface area, as a consequence, is much greater in the jejunum than in the ileum. The direct stimulatory effect of nutrients on the growth of the absorptive mucosa is believed to be responsible for the normal proximal to distal absorptive gradient; proximal jejunal villi are taller and have greater absorptive capacity because of abundant exposure to nutrients, most of which are depleted by the time intestinal chyme reaches the distal small intestine. The ileum of animals undergoing jejunectomy experiences marked hyperplasia when exposed to enteral nutrients. Similarly, when a segment of ileum is transplanted proximally and exposed to the jejunal nutrient environment, that segment becomes hyperplastic and takes on the morphologic and functional absorptive characteristics of proximal intestine (13). Conversely, a jejunal segment transposed to the ileal environment undergoes opposite changes and decreases its mucosal mass.
that reflect the functional compartmentalization of the small intestine. The thickness of the small bowel wall, the overall luminal diameter, and the prominence of the circular submucosal folds (plicae circulares) are all greatest in the proximal jejunum and decrease with progression through the ileum. Absorptive surface area, as a consequence, is much greater in the jejunum than in the ileum. The direct stimulatory effect of nutrients on the growth of the absorptive mucosa is believed to be responsible for the normal proximal to distal absorptive gradient; proximal jejunal villi are taller and have greater absorptive capacity because of abundant exposure to nutrients, most of which are depleted by the time intestinal chyme reaches the distal small intestine. The ileum of animals undergoing jejunectomy experiences marked hyperplasia when exposed to enteral nutrients. Similarly, when a segment of ileum is transplanted proximally and exposed to the jejunal nutrient environment, that segment becomes hyperplastic and takes on the morphologic and functional absorptive characteristics of proximal intestine (13). Conversely, a jejunal segment transposed to the ileal environment undergoes opposite changes and decreases its mucosal mass.
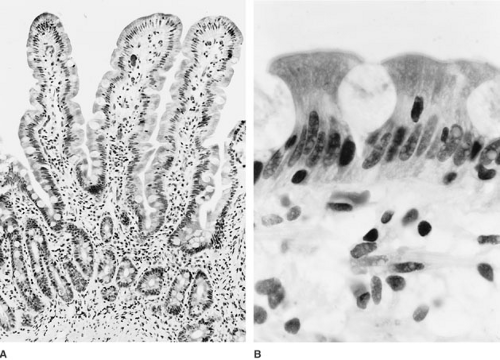
The mucosa is the innermost layer of the small intestine and is composed of three distinct layers: the muscularis mucosa, lamina propria, and surface epithelial cell lining (Fig. 74-2). As noted, the most striking feature of the small intestinal mucosa is the formation of villi, which are about 1.5 mm tall in the jejunum and progressively shorter through the ileum. Surrounding the base of each villus are several pitlike crypts, called the crypts of Lieberkühn, which average one-third to one-fourth the height of the villi and are the site of epithelial cell proliferation.
Cells that populate the epithelial layer differ depending on whether they overlay the villi, crypts, or lymphoid aggregates of Peyer patches. The most abundant cells in the crypt epithelium are undifferentiated columnar epithelial cells, with fewer goblet cells, enteroendocrine cells, and Paneth cells. These stem cells are responsible for cell renewal and can differentiate into all other cellular components. In addition, the crypt epithelial cells regulate water and ion secretion. Epithelial cells differentiate and develop mature digestive and absorptive function as they migrate from proximal crypt to villus tip. Structurally distinct M cells that are found in the epithelium overlaying Peyer patches appear to be important in antigen processing and presentation to immunocompetent gut lymphocytes.
The small intestine is in a perpetual state of turnover, with intense mitotic activity in the crypts, uniform migration of all cell types (with the exception of Paneth cells) luminally up the side of the villus, and eventual extrusion at the villus tip. As cells make this journey, they acquire the special structure and intracellular elements needed for their mature function, including the development of specific digestive and absorptive functions. Epithelial cell migration and maturation occur about every 3 to 5 days; the entire mucosal lining is replaced in 1 week.
Cellular Anatomy
Enterocytes are tall columnar epithelial cells that are responsible for absorption. These cells rest tightly on their basal laminae and are attached at the apical pole to adjacent enterocytes by tight junctions. In the jejunum, however, these tight junctions are actually permeable, and back diffusion of fluid and electrolytes into the lumen allows the luminal contents to remain isotonic even as the bulk of nutrients are absorbed. Conversely, in the ileum, the tight junctions are less permeable; there is less back diffusion resulting in increased concentration of luminal contents.
The luminal surface of the enterocyte has numerous membrane extensions, termed microvilli, which are about 1 mm tall. The microvilli (collectively known as the brush border) are in direct contact with the luminal contents and contain the membrane-bound digestive enzymes, transport proteins, and other elements necessary for nutrient absorption. The brush border can be damaged by multiple small bowel pathogens such as rotavirus or Giardia sp, leading to transient reduction in digestive enzyme function. Interposed between and lying on top of the microvilli is a glycoprotein coat called the glycocalyx or the “fuzzy coat.” Glycoproteins that make up this layer are resistant to removal by enzymatic activity (14). Fluid and electrolyte trafficking between cells is controlled by the junctional complexes between adjacent cells and the basolateral membrane. This portion of the cell membrane lacks the disaccharidases and peptidases present on the
apical membrane, but is rich in Na+-K+-ATPase, glycosyl transferase, and adenyl cyclase, all of which are involved in the major energy-dependent mechanisms that control solute and, thereby, fluid transport.
apical membrane, but is rich in Na+-K+-ATPase, glycosyl transferase, and adenyl cyclase, all of which are involved in the major energy-dependent mechanisms that control solute and, thereby, fluid transport.
Small Intestinal Motility
The two functions of small intestinal motor activity are to mix and propel ingested food, promoting effective digestion and absorption of luminal contents, and to sweep the small intestine clear of undigestible food particles, bacteria, and desquamated cells during periods of fasting. Coordinated peristalsis, consisting of contractions and relaxations, is regulated by the intrinsic activity of the smooth muscle together with the modulating actions of the autonomic nervous system and gastrointestinal hormones. The human enteric nervous system (ENS) consists of cell bodies and processes of the neural plexuses within the gut wall. It contains more than 108 neurons, which is roughly equivalent to the number of neurons in the spinal cord. Complexity of this neural control network has led to its being called the “little brain in the gut” (15).
During fasting, small intestinal motility is organized into a recurrent pattern of sequential periods, which together make up the MMCs. The MMCs have been called the “intestinal housekeeper” because they clear the small intestine of bacteria and undigestible food residue. Absence of MMCs in patients with pseudoobstruction is associated with bacterial overgrowth(16). MMCs consist of three phases. Phase 1 is a period of quiescence characterized by slow transit and maximal absorption of nutrients. It is followed by phase 2, a period of random, intermittent contractions similar to the normal postprandial pattern, designed for mixing rather than propulsion. Phase 3 is a brief period of high-amplitude contractions occurring at maximal frequency. Phase 3 can start anywhere from the lower esophageal sphincter to the distal jejunum, and propagates at a rate of 5 to 15 cm per minute toward the ileum. Phase 3 is also associated with an increase in intestinal secretions, which may aid in clearing the small intestine of bacteria and residue (17). As one complex reaches the terminal ileum, another starts in the proximal intestine. A complete MMC occurs every 90 to 300 minutes in adults and every 40 to 100 minutes in infants.
Ingestion of a nutrient meal disrupts the cyclic pattern of the migrating motor complexes (MMC), leading to a prolonged period of irregular activity similar to phase 2 during fasting. The fed pattern, however, has even fewer propagating contractions than phase 2 of the MMC and results in slow transit and more time for intraluminal digestion and absorption of nutrients in the proximal small intestine (18). The duration of the fed pattern correlates with the time of gastric emptying and is influenced by the characteristics of nutrients ingested. Fat meals are slower to empty the stomach and inhibit the MMC longer than isocaloric amounts of sucrose, which in turn inhibit the MMC longer than protein. Hormonal influences also play a role. Intravenous infusions of secretin, insulin, glucagon, cholecystokinin, or gastrin disrupt the MMC and induce a fed motility pattern, even in the absence of luminal nutrients. Neural control is also important because vagotomy inhibits the fed pattern, suggesting that vagal activity has a role in suppressing the fasting pattern (19). Local perfusion of anticholinergic compounds disrupts the MMC for a considerable distance proximal and distal to the perfusion site.
The ENS is comprised of the myenteric plexus (Auerbach plexus), situated between the longitudinal and circular muscle layers, and the submucosal plexus (Meissner plexus), located in the submucosa. The two plexuses contain networks of neurons, communicate through connecting nerve axons, and generate stereotypic patterns of electrical activity that regulate motility, secretion, and blood flow. These intrinsic patterns are essential, as illustrated by numerous animal models of extrinsically denervated intestinal segments that retain peristalsis and cycled MMCs. When the small intestine is divided into small segments by multiple transections and reanastomoses, each segment generates its own MMC, which cycles independently from other segments (20). Stimulation of motilin receptors by erythromycin, which ordinarily induces MMCs in adults, fails to induce similar motility changes in preterm infants who lack a mature ENS necessary for normal intestinal cyclic motor activity (21).
In the presence of a mature ENS, extrinsic neurogenic, chemical, and hormonal control is important in regulating small bowel motility. The small intestine is supplied by parasympathetic nerves from the vagus and by sympathetic nerves from the thoracolumbar spinal cord. Blocking the parasympathetic input by vagotomy or the administration of atropine reduces the duration of phase 2 of the MMC, and initiating an MMC by injections of motilin into the stomach requires an intact vagus (22). In contrast, extrinsic sympathetic nerves are important inhibitory efferents to the small intestine and mediate the disappearance of MMCs, as seen in patients who develop a postoperative ileus (23). Chemical excitatory substances that stimulate small bowel contraction include acetylcholine, tachykinins, and substance P. Likely candidates for postsynaptic inhibitors of contraction include vasoactive intestinal polypeptide (VIP), calcitonin gene-related peptide, nitric oxide, high doses of somatostatin, and β-endorphin. The peptide hormone motilin appears to stimulate MMCs that originate in the stomach, but MMCs that begin below the pylorus do not correlate with plasma motilin variation. Eating a meal produces an increase in the serum concentration of motilin (24).
Physiology of Water and Electrolyte Absorption
Osmotic differences of as little as 1.6 mOsm per L across the apical enterocyte membrane and of only an additional 0.7 mOsm per L across the basolateral membrane are believed to account for the transfer of water from the luminal space to the capillary space in the small intestine (25). Solute entry into the enterocyte to maintain this small osmotic gradient is achieved primarily by active transport processes. The apical (luminal) membrane of the enterocyte contains numerous active transporters of solutes, including glucose and amino acids, which transfer sodium in an obligatory process. The basolateral membrane contains an active transporter for sodium in the form of the Na+-K+-ATPase pump. Together, these processes maintain a small gradient of relative hypotonicity in the lumen, intermediate tonicity in the cell, and mild hypertonicity within the lateral intracellular space. Increasing hydrostatic pressure in this space helps push water across the freely permeable basal lamina and interstitial space into the capillaries of the lamina propria.
Ion transport across the small intestinal epithelium occurs by passive diffusion down a chemical concentration gradient or because of electrical potential differences, by solvent drag from the passive flow of solutes secondary to water flow, or by active transport carrier-mediated processes that are energy dependent and move ions against either an electrical or a chemical concentration gradient. In all segments of the small intestine, the final determinant of the driving force for all active ion transport is the Na+-K+-ATPase pump located along the basolateral membrane. This pump actively transports sodium out of the cell at the basolateral membrane in exchange for potassium. Absorption of sodium and other ions is regulated by the intestinal epithelium covering the villi, and secretion of chloride is predominantly handled by the crypt cells.
Chloride secretion occurs in intestinal crypt cells because of two mechanisms. Chloride is carried into the cell by a cotransporter located in the basolateral membrane, which results in the electroneutral transport of one sodium, one potassium, and two chloride molecules. Chloride carried across the basolateral membrane is then secreted from the cell through chloride-selective channels located in the apical membrane of crypt cells (26). These chloride-selective channels are generally closed,
but they can be opened by activating cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), or protein kinase C, or by calcium. cAMP is stimulated by cholera toxin, VIP, and prostaglandins (27). cGMP stimulation can result from Escherichia coli enterotoxin and calcium. As chloride is secreted across the apical membrane, sodium is lost through the paracellular pathway in response to the outwardly directed electrochemical gradient created by the chloride secretion.
but they can be opened by activating cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), or protein kinase C, or by calcium. cAMP is stimulated by cholera toxin, VIP, and prostaglandins (27). cGMP stimulation can result from Escherichia coli enterotoxin and calcium. As chloride is secreted across the apical membrane, sodium is lost through the paracellular pathway in response to the outwardly directed electrochemical gradient created by the chloride secretion.
Physiology of Nutrient Digestion and Absorption
Carbohydrates
Starches and oligosaccharides are the major form of carbohydrate in the human diet, but must undergo digestion to monosaccharides before they can be absorbed. Table 74-1 summarizes the extraintestinal and brush-border enzymes that participate in carbohydrate digestion to monosaccharides. A small amount of dietary disaccharide, and as much as 10% to 20% of starch, are not normally absorbed in the small intestine and pass into the colon to be fermented by colonic bacteria to short-chain fatty acids reabsorbed in the colon. This colonic reclamation of carbohydrate prevents loss of energy and the osmotic diarrhea that would result from carbohydrate malabsorption. It is also probably important in limiting diarrhea in patients with lactose intolerance, sucrase-isomaltase deficiency, and short bowel syndrome.
The most important enzyme in the digestion of starch is α-amylase, which acts on the interior α1-4 bonds of starch. Salivary amylase makes a minor contribution to overall starch digestion, except perhaps in neonates, because it is inactivated at gastric pH. Human milk, in contrast to cow milk, is rich in α-amylase activity (28). Pancreatic amylase activity is low in the first months of life, but in older children with normal pancreatic function, it is present in excess of normal requirements for starch hydrolysis. Therefore, starch digestion is essentially complete when intestinal contents reach the distal duodenum (29). Exposure of the duodenum to nutrient is the major stimulus for pancreatic amylase secretion. This effect is likely mediated through both the cholinergic nervous system and gut hormones because administration of a cholecystokinin receptor antagonist reduces meal-stimulated pancreatic enzyme secretion by 60%, and atropine causes nearly complete suppression of pancreatic enzyme secretion (30). Secretin, the major stimulant of pancreatic bicarbonate and fluid secretion, also promotes α-amylase secretion, but has less effect on lipase and little effect on pancreatic protease release.
TABLE 74-1 Extraintestinal and Brush-border Enzymes of Carbohydrate Digestion. | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Fats
Figure 74-3 provides an overview of the major steps of lipid digestion and absorption. Despite the complexity of the process, absorption from the lumen into the enterocyte and then to the lamina propria requires only about 12 minutes, and beyond infancy, more than 95% of intraluminal fat is normally absorbed. Triglycerides, which comprise about 90% of dietary fat intake, are emulsified into tiny particles and stabilized by other dietary components, such as phospholipids and polysaccharides or endogenous bile salts. Bile salts are important in solubilization and in the promotion of pancreatic lipase activity, but 75% of dietary triglyceride can be absorbed without bile salts. Cholesterol and fat-soluble vitamin absorption are affected more severely by bile acid deficiency than is triglyceride absorption.
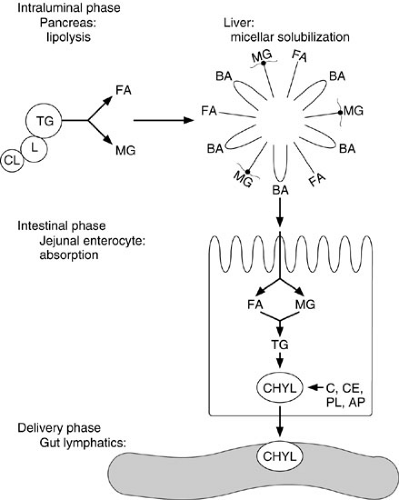 FIGURE 74-3. Overview of the major steps of lipid digestion and absorption. (1) Lipase (L) and colipase (CL) bind to the triglyceride (TG) droplet. (2) Intraluminal lipolysis of TG to fatty acids (FA) and monoglycerides (MG). (3) Formation into micelles with bile acids (BA). (4) Absorption of the FA and MG into the enterocyte. (5) Reesterification into TG and formation of chylomicrons (CHYL) made up of cholesterol (C), cholesterol ester (CE), phospholipid (PL), and apoproteins (AP). (6) Secretion of CHYL into intercellular spaces and gut lymphatics in lamina propria.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|