INTRODUCTION
History
The technologic advances in minimally invasive surgery, specifically gynecologic surgery, over the past 50 years are staggering. Using monocular laparoscopes with continuous carbon dioxide insufflation of the peritoneal cavity, most gynecologists in the United States in the early 1970s were performing diagnostic laparoscopy and tubal sterilizations. Within a short period of time, video laparoscopy allowed a rapid expansion in procedures performed by the minimally invasive approach. Salpingectomy, oophorectomy, myomectomy, resection and ablation of endometriosis, hysterectomy, and a long list of other procedures, are now commonly performed. Until recently, most technologic advances in laparoscopy have involved video equipment, instrumentation, and newer energy sources. A combination of astounding technologic advances has resulted in the introduction of robotic or robotically assisted surgery in gynecology, and astute, aggressive marketing to physicians and the public has resulted in the rapid assimilation of robotic surgery into today’s surgical practices. However, controlling the application of robotic technology and teaching the procedures seem to lag behind the marketing.
According to the Oxford Dictionary, “robot” is derived from the Czech word robota meaning “forced labor.” The term was introduced in Karel Čapek’s play “Rossum’s Universal Robots” in 1920. A robot is defined as a machine capable of carrying out a complex series of actions automatically, especially one programmable by a computer. The history of robotic surgery is best described as a convergence of research performed in multiple locations in the 1980s. Expanding on the technique of stereotactic brain biopsies, Kwoh and colleagues modified an industrial robot, the Unimation PUMA (Programmable Universal Machine for Assembly) 200, for the biopsy of brain tumors in 1988. In 1991, Davies et al., at the Imperial College of Science, Technology, and Medicine in London reported the use of a modified PUMA 560 robot, which later became PROBOT, for transurethral resection of prostatic tumors. During the 1980s and early 1990s, researchers at the National Aeronautics and Space Administration Ames Research Center were working on virtual environments while researchers at the Stanford Research Institute were working on robotics and a telemanipulator system for hand surgery. As described in a review by Satava, the Department of Defense and the Defense Advanced Research Projects Agency funded research directed toward the concept of telepresence surgery for combat casualty care. This culminated in the demonstration of a telepresence vascular anastomosis in the swine model in 1996 by Bowersox and telesurgical laparoscopic cholecystectomy in 1998 by Himpens. In 1993, Yulun Wang, PhD, formed Computer Motion, which developed a robotic camera holder for laparoscopy called Automated Endoscopic System for Optimal Positioning (AESOP) and ultimately created an integrated robotic system that attached to the operating table called ZEUS. In 1995, Frederic Moll, MD, acquired the license for the telepresence surgical system and created Intuitive Surgical. In 2003, Computer Motion and Intuitive Surgical merged into a single entity, Intuitive Surgical. Presently, da Vinci is the only robotic surgical system approved by the U.S. Food and Drug Administration (FDA) (Intuitive Surgical, Sunnyvale, CA). As a result, any reference to robotic surgery or robotic platform in the remainder of this chapter pertains to the Intuitive, da Vinci system. This is not meant to be an endorsement of this or any other product; it simply reflects the fact that there are no other commercially available robotic platforms at present.
Advantages and Disadvantages
The November 2009 American Congress of Obstetricians and Gynecologists Committee Opinion concerning types of hysterectomy concluded that vaginal hysterectomy is the approach of choice whenever feasible, on the basis of its well-documented advantages and lower complication rates. Laparoscopic hysterectomy is an alternative to abdominal hysterectomy for those patients in whom vaginal hysterectomy is not indicated or feasible. “The experience with robot-assisted hysterectomy is limited at this time; more data are necessary to determine its role in the performance of hysterectomy.” The advantages of robotic surgery include those experienced by the patient, the hospital, and by the surgeon. The advantages to the patient include smaller incisions compared to laparotomy, shorter hospital stay, less blood loss, and a more rapid return to normal activity. Laparoscopic surgery provides the same benefits; but because of technical considerations noted below, the range of surgical procedures is greater with robotic surgery. The advantages to a surgeon include enhanced visibility with a threedimensional (3D) view, enhanced image magnification, and stable camera platform devoid of first-assistant fatigue that can result in an unstable field of view with camera motion. The instruments utilized by the surgeon are wristed to allow a full range of motion and angles similar to an open procedure. In distinction to conventional laparoscopy, which relies on a fulcrum effect and requires movement of the handle of the instrument in the opposite direction to the desired movement of the tip, robotic surgery allows hand and instrument movement in the same direction. Surgeon hand tremor is minimized, and the ratio of hand motion to robotic instrument motion can be adjusted. Sarlos reported that fatigue and back discomfort may be reduced by a more ergonomically correct position at the surgeon console. This benefit may be the greatest during longer cases. Robotic suturing may be considerably easier for most surgeons compared to laparoscopic suturing in studies reported by Andenberg and Chandra. For instructing resident physicians and fellows-in-training, the video monitor on the vision cart allows the educator to draw on the monitor that can be seen by the trainee at the console. Sonographic or computed tomographic images from preoperative studies can be projected onto the surgeon console screen for viewing during surgery. The newest da Vinci Si model allows surgeons at two separate consoles to operate simultaneously on the same patient, or it allows a mentor to intervene during a trainee’s procedure. The potential advantage to the hospital or institution is a shorter patient hospital stay for procedures that historically resulted in a longer stay if performed by laparotomy. Potential disadvantages to the patient include a longer operative time and facial edema from protracted Trendelenburg position.
The major disadvantage to all parties is the cost of the robot, associated instruments, drapes, and maintenance contracts. The cost of a da Vinci robotic system varies between $1,000,000 and $2,300,000. Wristed robotic instruments, which have a limit of 10 uses, cost approximately $1,300.
For point of reference, the 2011 purchase price paid by one of our community hospitals for typical laparoscopic instruments utilizing different energy sources ranges from $350 to $450. Annual maintenance costs for the robotic system are estimated at $150,000 (Intuitive Surgical, Second Quarter Investor Presentation,
http://phx.corporate-ir.net/phoenix. zhtml?c=122359&p=irol-IRHome).
Because of the size of the entire robotic system, larger operating rooms (ORs) are required for optimal efficiency and may add expense to the institution. Size and position of the robotic arms when docked may cause difficulty for the bedside assistant when passing sutures, retracting tissues, or manipulating the uterus with a vaginal manipulator.
From the surgeon’s standpoint, the major disadvantage is the lack of haptic or tactile sense with the instruments. Those surgeons accustomed to blunt dissection of tissues will be required to enhance their sharp dissection skills and rely on anatomic landmarks. Those in residency training may suffer from diminished experience with traditional open procedures and operative technologies as more and more gynecologic surgical procedures are done using minimally invasive and robotic techniques.
ROBOTIC TRAINING
In 2007, Javier Magrina explained that robotic surgery “… is nothing more than an enhancement along the continuum of laparoscopic technologic advances and represents only the beginning of numerous more forthcoming advances.”
Obstacles and Challenges to Teaching and Learning
Currently, there is no standardized training model or validated method to teach robotic surgery. Two notable obstacles to teaching the application of this new technology result from the unique features of robotics. Until recently, a single surgeon controlled the robotic operation without the use of a second set of independent controls for the assistant or trainee. The teacher/instructor had no ability for immediate hands-on correction as he or she would have had with traditional teaching. Thus, potential patient safety issues become a concern with implementation of robotics. The situation is likened to a student pilot on final approach without the flight instructor being able to take control of the airplane’s pitch and power controls to avoid a potentially dangerous landing. The robotics instructor must correct the trainee with prompt, understandable, and verbal commands. However, dual consoles are now available, although they are expensive and not widely available. These dual consoles allow cosurgeons to work on the same patient with three moving instruments controlled by the cosurgeons in contrast to two moving instruments with a single console. Additionally, a mentor and a trainee can operate on the same patient simultaneously. The single-surgeon console in use at most facilities is a “resident-unfriendly” platform. The resident initially functions as a table-side assistant or observer, which is quite different from the usual hands-on learning experience. Hanly suggested that wider availability of mentoring consoles will likely improve resident and nonresident learning experiences and enhance patient safety. The telestration feature enables the teacher/mentor to point, draw, or illustrate directly on the video touch screen monitor as the surgeon sees this on the surgeon console screen in real time.
Another obstacle to learning robotic surgery is the lack of tactile sensation and feedback to the surgeon. Palpation of the tissues is not translated to the surgeon in the same way as one “feels” the tissues with open procedures or conventional laparoscopy. This lack of tactile sensation or haptics presents an initial challenge to learning until one is conditioned to develop a “sensation of feel” on the basis of the visual cues as proposed by Hagen and colleagues. This may be likened to an experienced pilot transitioning to flying a remote-controlled drone aircraft from a virtual cockpit far from the area of flight. Despite these obstacles, Dharia and Falcone, Stefanidis et al., and Suh et al. suggest that the robotic features of instrument motion comparable to open procedures, tremor reduction, 3D high-definition image, wristed instrument articulation, and downscaling movements have made more difficult advanced laparoscopic surgical cases with steep learning curves easier to learn and apply to patient care.
Training Methods
Initially, most robotic training regimens were developed and sponsored by Intuitive Surgical, Inc., the maker of da Vinci Surgical System. The manufacturer has been active in developing training methods and recommendations to introduce the techniques of robotic surgery to potential customers. However, with increased experience of surgeons and institutions, locally developed training protocols have placed less emphasis on the manufacturer to provide training. Initial training protocols incorporated a combination of “on-site” (hospital) training, “off-site” (laboratory) hands-on courses, as well as Internetbased modules and surgical videos. All OR team members require basic training on mechanical and technical aspects of robotic system functions (system training). This specifically includes learning the components of the system, how to dock, set up, and insert instruments, implement safety features, and troubleshoot malfunctions. The second aspect of training deals with acquiring the technical skills to perform surgery (procedure training). Training modules and videos may be accessed at http://www.davincisurgerycommunity.com.
Since there is only one commercially available robotic surgical system available in the United States today, the technical system training is still largely provided by the manufacturer. This system training can be completed with the Internet-based Intuitive Surgical curriculum including system overview, setup/docking, surgeon console functions, and safety features. The setup module instructs on port placement, camera arm positioning, instrument arm positioning, docking, endoscope insertion and removal, and instrument insertion and removal. The surgeon console module instructs on system setup and vision, ergonomic, and instrument control. The safety features module teaches how to troubleshoot fault messages, emergency switch location and use, energy source control, and procedure conversion to open or standard laparoscopy.
Procedure training is surgeon and procedure specific and directed at acquiring the needed technical skills. For the gynecologist/pelvic surgeon, a sample of the procedures performed robotically include hysterectomy, adnexal surgery, lymphadenectomy, radical hysterectomy, myomectomy, sacrocolpopexy, Burch retropubic urethropexy, endometriosis resection, and ureteral and fistula repairs. Commercially produced and Web-based surgical videos provided by surgeons are available. Inanimate simulator training, live animal models (pig lab), and case observation have been required of surgeons learning robotic techniques before being proctored by an experienced surgeon. After certification of completion of both system and procedure training, experienced gynecologic surgeons desiring to obtain robotic surgical privileges are commonly proctored at their home institution with a minimum of two to five cases before independent-use privileging is granted. Because of the high expense of animal laboratory courses ($3,000 per one to five trainees), these may not be feasible for some residency/fellowship training programs. Hoekstra and Geller have reported on nonstandardized methods developed to achieve didactic
and procedural training within their institution. Completion of a procedure training program described by Geller and associates at the University of North Carolina is required before residents or fellows performing robotic surgery. This “System Skills Practicum” includes developing proficiency in four surgical skills: (a) “manipulation drill” that requires transfer of rubber rings from tower to tower to learn wristed instrument movements and camera manipulation (clutching), (b) “dissection drill” that requires dissection of a vessel encased in gelatin using scissors and grasping instruments, (c and d) “suturing drills.” The “suturing drills” are timed and involve reapproximating linear and jagged lacerations on an inanimate model to teach needle-driver use and intracorporeal knot tying with camera and instrument clutching. These suturing drills are the most difficult to master. Surgical training programs such as residency and fellowship also typically require involvement in a minimum number of case observations in addition to working as first assistant with progressive hands-on experience.
The simulation training module connecting to the da Vinci Si model functions the same as a flight simulator or video game box. The surgeon works directly at the console on a series of exercises to learn and maintain proficiency skills in manipulation of the robotic controls. The simulation unit scores and records each exercise to allow quantification of progress. Manipulation skills of the wristed instruments, camera control, clutching, dissection, energy control, and needle driving are performed in various combinations. Surgical facilities responsible for credentialing may be able to utilize this module to assess surgeon skill retention and possibly to maintain privileges.
Does Previous Experience with Operative Laparoscopy Matter?
Several studies have evaluated experienced surgeons and inexperienced/novice surgeons with regard to skill development when learning simple and complex robotic techniques. Stefanidis and Suh found that previous experience as an assistant surgeon on robotic surgical cases shortens the learning curve, and all trainees have lessened physical demands and decreased workload with improved accuracy and precision in comparison to standard laparoscopic techniques. They also reported that surgical novices seem to learn difficult tasks such as robotic suturing more easily than experienced surgeons. Interestingly, other high-intensity hand-eye coordination activities, such as prior high-volume video game experience, were found to have a negative impact on learning robotic suturing in a study by Harper. Overall, it appears that lack of previous laparoscopic experience does not seem to be a major obstacle in learning the technical aspects of the robotic platform.
Experience with Various Energy Sources
As with standard operative laparoscopy, the surgeon performing robotic surgery should be familiar with the various energy sources, as the power settings and depth of thermal spread may be new or different from what they are accustomed to with standard laparotomy, vaginal, or laparoscopic procedures. Energy sources used with the da Vinci system include bipolar cautery, monopolar cautery (scissors), PlasmaKinetic (PK), and harmonic energy sources. If bedside assistants will be operating conventional laparoscopic energy sources, adequate education and experience are required.
What Is the Learning Curve for Robotic Surgery?
Operating time may represent an indirect measure of the learning curve for a specific surgical procedure. An efficient OR team should lead to decreased operating times. Because of the significant technical training robotic surgery requires of the entire surgical team, safety and efficiency in robotic surgery require a well-trained, experienced team, which is used to working together. The importance of the same team working together frequently with the same surgical procedures cannot be overemphasized. Several components contributing to operating times include OR personnel, surgeon, and patient factors. First, efficiency is dependent upon the OR personnel for room turnover, equipment processing and setup, and assisting the surgeon in positioning and draping the patient. Second, surgeon factors depend on his/her learning curve or experience performing the particular operation. As with any other surgical method, operating times decrease and technical proficiency increases as the frequency and volume of cases increase to a certain number. Third, patient-dependent factors that increase the difficulty of the surgery, such as morbid obesity, previous surgeries, and presence of extensive adhesions, are to be considered. The patient factors should be considered especially when selecting initial cases and will depend on the surgeon’s experience and skill level. A study by Hoekstra and colleagues found a learning curve of 5 to 10 cases to demonstrate proficiency in simple hysterectomy, cuff closure, and pelvic lymph node dissection for gynecologic oncology fellows. Useful methods of teaching were verbal feedback, telestration teaching, and demonstration of a portion of the procedure after a first attempt by the fellow. Looking at hysterectomy performed in a community-based practice, Lenihan and associates found that surgical time stabilized at a total time of 95 minutes after 50 cases.
In a recent study of gynecologic surgeons learning robotic surgery skills, Woelk et al. concluded that “surgical proficiency” with the new techniques required performing many more robotic procedures than previously thought. A total of 325 robotic hysterectomies were done by eight different gynecologic surgeons over a 3-year period at the Mayo Clinic. Operative time and intraoperative and postoperative complications were recorded for each surgeon, and results were analyzed in 6-month blocks of time. Operative time and postoperative length of hospital stay declined significantly over the 3-year study, but complications did not change significantly, although there was a trend toward fewer complications as surgical experience accumulated.
The risk of complications and length of surgery continued to decline and did not level off for any of the surgeons over the 6 to 36 months of evaluation, which included up to 150 hysterectomies for one of the surgeons. None of the surgeons monitored had a complication rate of greater than double the local benchmark rate for abdominal hysterectomy (11.4%), but only one surgeon, the most experienced, was able to achieve a complication rate of less than the abdominal hysterectomy benchmark (5.7%)—and this was finally achieved after 91 robotic hysterectomies. This was the only surgeon who actually did over 90 robotically assisted hysterectomies during the course of the study.
Credentialing Requirements
Credentialing and granting hospital privileging vary throughout the United States. Individual institutions may rely on recommendations from local or national experts for guidelines regarding robotic surgical privileges. The robotic platform is a complex surgical tool, and surgeons should be expected to be specifically credentialed to perform each operation, for example, hysterectomy, using robotic techniques. Credentialing in basic operative laparoscopic skills makes sense, as robotics is an adaptation of laparoscopy. However, advanced laparoscopic skills are not necessarily required or helpful for the robotic surgeon. For experienced surgeons, two to five proctored robotic surgical procedures are usually required
before independent robotic privileges are granted. Residents and fellows will likely have a more protracted exposure to robotics and may require performance or significant involvement in more cases before showing proficiency.
In our institution, credentialing criteria for robotic surgery include core privileges in the specialty (gynecology, urology, general surgery, etc.) with documented attendance at a basic certified course in robotics and observation of a minimum of two full robotic cases before being granted temporary privileges. Documentation of robotic training during residency or fellowship may satisfy the above requirements. Before performing proctored procedures, the surgeon must perform a minimum of two documented simulated runs with OR staff and the robotic instrument specialist. After this, two proctored cases are observed by a physician with robotic privileges. Surgeons are then able to operate independently and must perform a minimum of eight procedures within the next year to maintain robotic privileges. The Department of Obstetrics and Gynecology places the surgeon in a period of focused review for ongoing evaluation of outcomes and patient safety.
How Will Resident Training Be Altered with the Advent of the Robotic Platform?
As the technologic advances in minimally invasive surgical procedures increase, the demands placed on resident training are in constant flux. During the late 1980s, vaginal, simple laparoscopic, and open surgery progressed to incorporate more advanced hysteroscopic and laparoscopic techniques. Eventually, this led to the more common use of total and subtotal laparoscopic hysterectomy. Twenty years later, in an era of robotic surgery, the gynecologic surgeon in training has a wider spectrum of procedures to master in the same 4-year time frame. Will resident physicians graduate with an adequate number of each type of hysterectomy to be proficient? Will it become rare to perform the traditional open abdominal hysterectomy? Will the vaginal hysterectomy be utilized less frequently? How do we teach patient selection for robotic surgery? While robotic surgery may replace abdominal hysterectomy for many indications, vaginal hysterectomy is associated with low morbidity, short operating time, rapid postoperative recovery, and low cost, so it should remain the technique of choice when technically possible.
With regard to resident training in hysterectomy, in the United States, obstetrics and gynecology residents perform an average of 120 hysterectomies during their training. This is an overall decrease in the total number of hysterectomies compared to years past, with a notable decrease in the number of vaginal hysterectomies as reported by Pulliam. Now, with robotic training, there is the potential for a significant decrease in the number of abdominal hysterectomies performed by the average gynecologic surgeon as well as the resident-in-training. In our community, we found that abdominal hysterectomies decreased significantly after robotic surgery became available, but no decrease in vaginal and/or laparoscopic-assisted vaginal hysterectomy was detected.
ROBOT COMPONENTS AND INSTRUMENTATION
Although the da Vinci robotic system is currently the only FDAapproved robotic system for surgery, there are three models currently in use: the original standard unit, the S model, and the Si model. The models vary in instrument length, camera capability, presence of a third operating arm, and the availability of high-definition video capability. Each system consists of the patient-side cart, the surgeon console, and the vision cart/console.
Vision Cart
A 12-mm endoscope (
Fig. 17.1) that is passed through the camera port in the patient is internally composed of two 5-mm lenses attached to the camera head, all of which are on the sterile field. The camera cables are passed off the field and attached to the vision system, which processes the images from the two 5-mm lenses and produces the 3D stereoscopic view. In addition to the vision system, the vision cart/console also contains the light source, heated carbon dioxide insufflator, and cautery unit or another energy source (
Fig. 17.2).
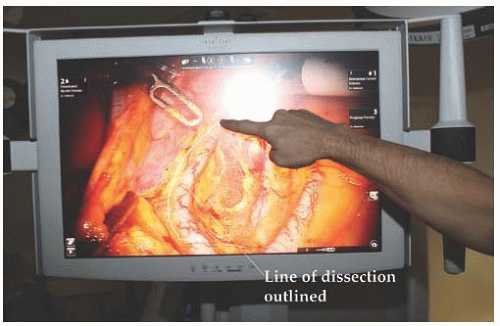
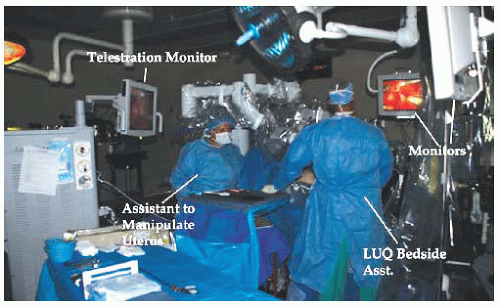
The vision console is usually equipped with a video monitor that provides a 2D view of the operative field and allows telestration. The touch screen monitor allows the table-side assistant, educator, or colleague to draw on the monitor, which projects over the field visualized by the surgeon at the surgeon console. This allows for education of the surgeon, trainee, or members of the surgical team (
Fig. 17.3). The vision cart is mobile and pulled adjacent to the operating table once the surgical procedure has begun.
Patient-Side Cart
The patient-side cart (
Fig. 17.4) is placed immediately next to the operating table by either straight docking between the patient’s legs or side docking at the level of the right or left hip. The standard system was designed with a camera arm and two operating arms. The S and Si systems have three operating arms and a camera arm. Positioning of the camera arm and operating arms varies with the procedure to be performed. The arms are attached to laparoscopic ports through which the wristed instruments or camera is placed. Without a second surgeon console, only two of the robotic operating arms are
capable of movement at any point in time. If the third arm is docked, one of the arms can serve as a retractor while the surgeon utilizes the other two arms for surgery. The surgeon may alternate or toggle between the available robotic operating arms. Instruments are interchangeable between all three operating arms. Most surgeons will use monopolar scissors for dissection in one arm and a grasper utilizing bipolar cautery, PK, or harmonic shears for hemostasis in the opposite hand.
Surgeon Console
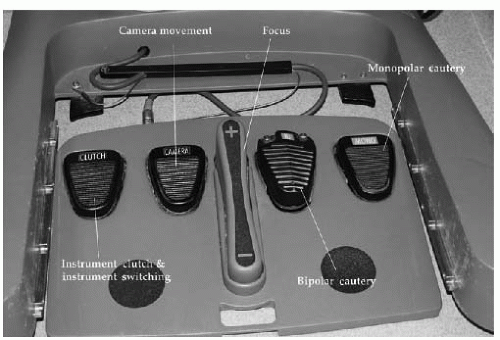
The surgeon console (
Fig. 17.5) is composed of a stereoscopic viewer through which the surgeon views the surgical field: two master controllers used to manipulate instruments and move, rotate, and focus the camera and a series of foot pedals. Foot pedals on the right side are used to activate the energy sources for the two operating instruments. A camera pedal on the left side allows movement of the camera. The S system has a pedal to focus the camera and a separate pedal on the left side to switch between two operating arms (
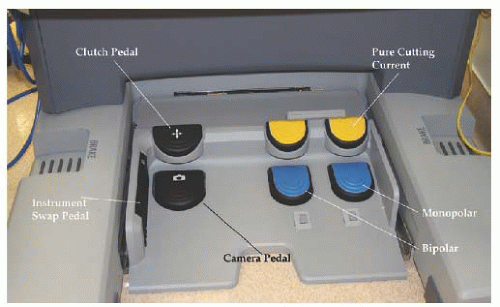
Fig. 17.6). The Si system does not have a foot pedal to focus the camera, as that function is accomplished with the master controllers. A toggle switch is built into the side of the Si unit that is foot-activated to alternate operating instruments (
Fig. 17.7). As mentioned earlier, the Si system allows a second surgeon at a separate console to
operate in parallel with the primary surgeon by controlling the third operating arm. In addition, the Si system allows visualization of radiographic images by the surgeon in the surgeon console display. While the surgeon sees intraoperative images in high definition and three dimensions, the OR staff sees conventional images on the accessory monitors. The Si unit also allows for a teaching/simulation unit to be attached and operated from the surgeon console.
Instruments
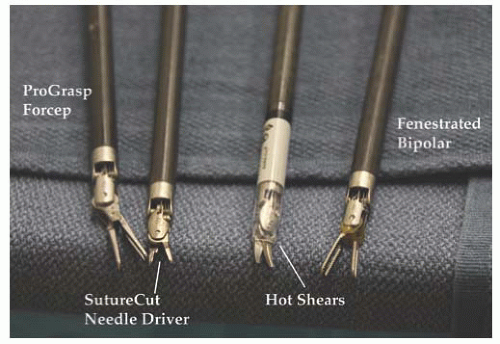
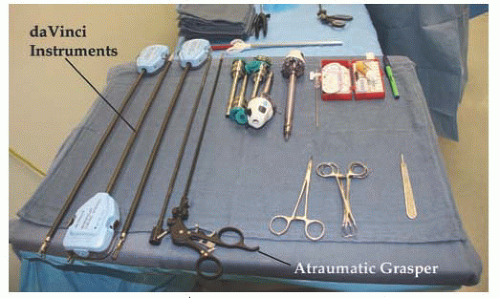
A variety of interchangeable instruments (
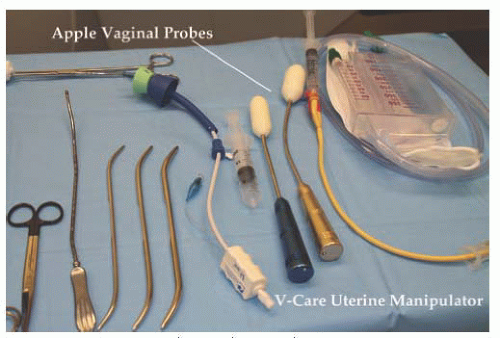
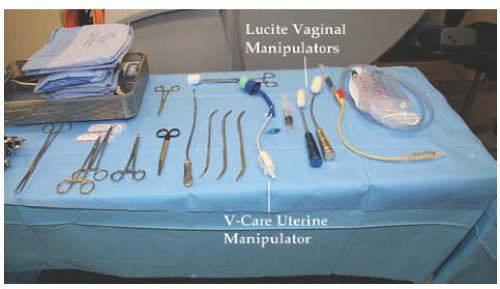
Fig. 17.8) specific to robotic surgery are available for use. As mentioned previously, most surgeons will utilize scissors with monopolar cautery in one operating arm and a grasper with attached energy source for hemostasis in the opposite arm. A fenestrated grasper is available for the third arm. Additional instruments include vascular forceps, a single-tooth tenaculum, a monopolar spatula, and several types of needle drivers. Each da Vinci-specific instrument may be reused up to 10 times. The table-side assistant may use a laparoscopic atraumatic grasper, irrigator/aspirator, energy source, and needle driver through the right or left upper quadrant assistant port. If a hysterectomy is performed for reasons other than cervical cancer, most surgeons will utilize a uterine manipulator. Two popular manipulators are the RUMI system (CooperSurgical, Trumbull, CT) with Koh ring or a VCare Manipulator (ConMed EndoSurgery, Utica, NY). If manipulating the cervix is contraindicated, the Apple vaginal probe (Apple Medical Corp, Marlborough, MA) is helpful (
Fig. 17.9).
Energy Sources
The use of an energy source for tissue dissection and cutting will usually involve monopolar electrocautery with a da Vinci scissor or spatula. Bipolar cautery may be applied with a fenestrated forceps or Maryland dissector. Additional energy sources used with robotic instruments include Harmonic ACE (ultrasonic shears) (Ethicon Endo-Surgery, Cincinnati, OH) and the Gyrus PK dissecting forceps (advanced bipolar) (Gyrus ACMI, Maple Grove, MN).
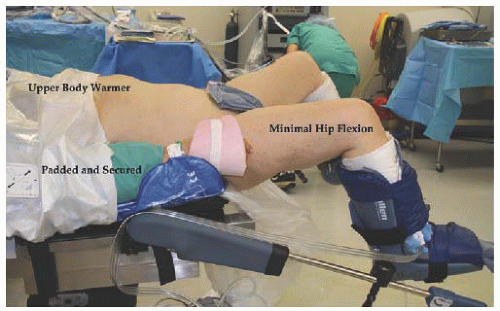
OR AND TABLE SETUP
If possible, the largest available OR should be assigned as home for the robotic system. Sufficient room must be allowed for the operating table to be positioned for a variety of cases involving the pelvis, upper abdomen, thorax, and head and neck region. The patient-side cart and vision cart are mobile and parked away from the operating table until needed. Once needed, they are positioned adjacent to the operating table for surgery. Gynecology cases will involve two operating table setups: one for placing vaginal instruments and one larger table for the abdominal portion of the procedure (
Figs. 17.10 and
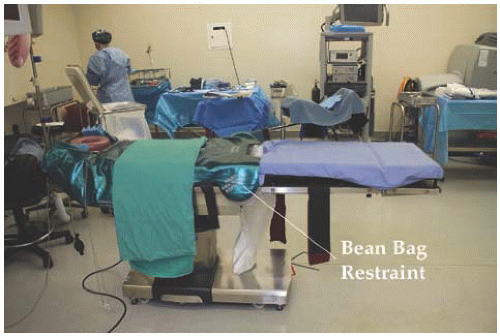
17.11). Most operating tables currently in use will allow for patient weight in excess of 600 pounds and 30 degrees of Trendelenburg position. To avoid movement of the patient in steep Trendelenburg position, the table should be equipped with a warmed gel pad allowing for friction with the patient’s bare skin or a beanbag device, which molds to the patient (
Figs. 17.12 and
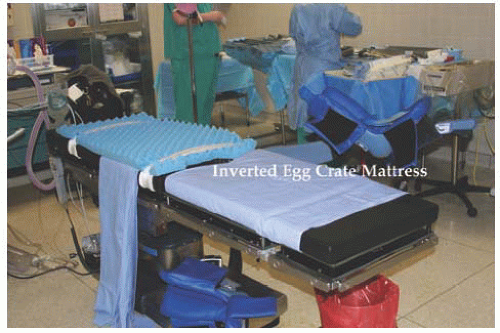
17.13). Another option is an inverted egg
mattress taped to the bed for bare skin contact (
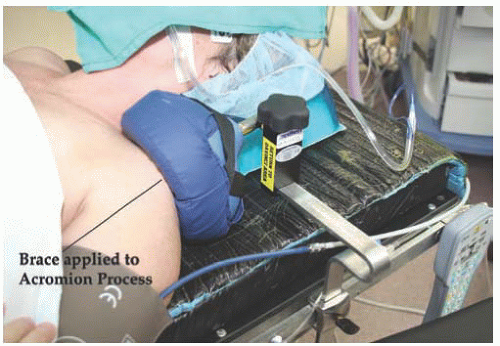
Fig. 17.14). The patient’s arms are padded with foam, tucked to her side, and secured with bedsheets. Shoulder braces may be used with care to prevent movement of the patient (
Fig. 17.15). If not positioned carefully, the shoulder braces may put the patient at risk for nerve injury. Adjustable stirrups in standard and bariatric sizes are needed to allow placement of vaginal instruments. With motion of the camera arm, there is risk of contact with the patient’s face, resulting in injury. Padding of the patient’s face will be addressed in the anesthesia section.
Anesthetic Considerations
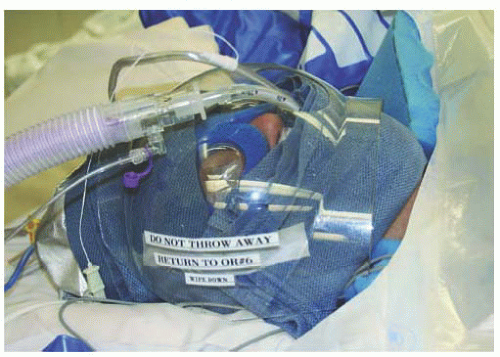
Anesthetic concerns regarding OR procedures for robotic surgeries include the use of steep Trendelenburg, tucking and padding both arms, covering both legs, and padding and covering the patient’s face. Airway pressures may be elevated due to increasing patient size and steep Trendelenburg positioning. Intra-abdominal pressures created by carbon dioxide insufflation and intestines resting on the diaphragm can create an anesthetic challenge. Over time, surgeons and anesthesiologists have become more comfortable with these challenges. When our program started, all patients had two large bore intravenous (IV) sites, and in many cases, an arterial line was placed. Currently, invasive monitoring is seldom needed, and in most cases, a single IV site is utilized. We have made an effort to assess all patients in the preanesthesia clinic several days prior to surgery, therefore providing the safest possible surgery and anesthetic experience. Many gynecologic procedures will involve placing a laparoscopic port in the left upper quadrant of the abdomen for use by the first assistant. We routinely place an orogastric tube to decompress the stomach and avoid gastric trauma from port placement. An important anesthetic consideration is knowing that gastric secretions may track along the orogastric tube to fill the mouth or stain the patient’s face. As mentioned earlier, the camera arm of the robot is in constant motion and may come in contact with the patient’s face, especially when the camera port is placed well above the umbilicus or a 30-degree, downward lens is used. To prevent trauma, eye shields are placed on the patient; followed by foam padding; and then a face shield allows egress of the endotracheal tube, esophageal stethoscope, and orogastric tube (
Fig. 17.16). In cases of lymphadenectomy, the IV fluid rates are kept at a minimum by anesthesia to avoid venous distention.
The Dedicated OR Team
As the surgeon is sitting at the surgeon console without patient contact, he or she relies exclusively on the dedicated OR team to be the hands and eyes at the table. Dedicated
implies two definitions: team members who are personally committed to the most efficient and safe robotic procedures possible, and OR management that will assign the same skilled staff for all cases rather than rotating multiple OR staff. Members of our team include a circulating registered nurse familiar with the variety of robotic procedures, driving the patient-side cart and troubleshooting any problems with console error messages and instrument needs. In addition, we utilize two table-side assistants, one of whom will provide retraction, pass instruments or suture, irrigate the field when needed, and address any conflicts between robotic arms. The second table-side assistant is responsible for uterine manipulation and specimen retrieval (
Fig. 17.17). Both assistants drape the upper abdomen while the surgeon places the uterine manipulator. The two bedside assistants may be surgical scrub technicians, certified surgical first assistants, physician assistants, nurse practitioners, or resident physicians. The most efficient operations will be associated with the most experienced table-side assistants.
The Surgeon
In addition to operating the instruments, the surgeon has several other important perioperative responsibilities. The surgeon is responsible for assuring a reasonable period of time for the initial learning curve, appropriate patient selection, preoperative preparation of the patient, and calm leadership of the rest of the team. There are several possible definitions of the “learning curve” for robotic surgery. It may be defined as the length of time or number of cases to reach a predictable operating time, the fewest complications, or lowest blood loss. It is not in the patient or surgeon’s best interest to start with the most complicated procedures. Dealing with a morbidly obese patient, multiple previous abdominal procedures, large fibroid tumors of the uterus, or severe pelvic endometriosis while still learning to effectively manipulate the robotic instruments will result in more complications, unnecessarily long surgical times, and surgeon frustration. Accommodating to the use of different surgical instruments and the lack of haptic sensation is best dealt with by scheduling less complicated cases at the onset of training. Once appropriate patient selection has been completed, the surgeon should explain the aspects of surgery and provide detailed postoperative instructions in advance of the surgery. We have been emphatic about the need to avoid straining or Valsalva maneuvers in the first 2 weeks postoperatively and avoiding any vaginal sexual intercourse for 6 weeks following surgery. On the basis of the type of procedure planned, the surgeon will decide on placement of the camera port, first-assistant port, and two or three operating ports. The operating table will be positioned to allow either side docking or docking between the patient’s legs. The surgeon will place the uterine manipulator and, in many cases, place sutures in the cervix if a hysterectomy is planned. The surgeon will tell the table-side assistant which instruments and energy sources will be needed. Once the patient is in Trendelenburg position and ports are in place (
Figs. 17.18 and
17.19), the robot is docked and instruments are placed.
At that point, the surgeon assumes control of the surgeon console and initiates the internal portion of the procedure. Once the procedure is complete, the robot is undocked, the abdomen is deflated, and port site skin incisions are closed. Using noncutting trocars, for the 8-mm and 12-mm ports, we have not routinely closed the fascial incisions. All incisions are injected with local anesthetic.
Postoperative Care
One of the greatest advantages of minimally invasive surgical procedures, whether laparoscopic or robotic assisted, is the early mobility and dismissal of patients. At the completion of the procedure, if renal function is normal and the patient is well hydrated, IV ketorolac is given before leaving the OR. A 1- to 2-L bolus of intravenous fluids is given in the recovery room, and the Foley catheter is removed prior to discharge from the recovery room. Regular diet, oral analgesics, and IV ondansetron are part of the routine postoperative orders. Patients are discharged home once they are able to tolerate a liquid diet, demonstrate adequate pain control, and void without difficulty. More than 50% of patients can be dismissed the day of surgery and more than 95% dismissed in less than 24 hours after the surgery is complete.
GYNECOLOGIC PROCEDURES PERFORMED ROBOTICALLY
Although many types of gynecologic procedures have been performed, we will focus on the five most common: robotic hysterectomy, robotic hysterectomy and staging lymphadenectomy, robotic radical hysterectomy, robotic myomectomy (RM), and robotic sacrocolpopexy (RSCP). The first report of uterine horn reanastomosis in an animal model was reported by Margossian in 1998, followed by a pilot study by Falcone and colleagues applying robotic surgery for tubal reanastomosis in humans in 2000. In 2002, Diaz-Arrastia and colleagues at the University of Texas Medical Branch in Galveston reported 11 patients undergoing robotic hysterectomy and bilateral salpingo-oophorectomy. Blood loss ranged from 50 to 1,500 mL, and operative times ranged from 4.5 to 10 hours. By 2006, Reynolds and Advincula reported their experience with 16 patients: 12 undergoing robotic-assisted laparoscopic hysterectomy and 4 treated with supracervical hysterectomy. Blood loss ranged from 50 to 300 mL, and operative times ranged from 170 to 432 minutes. Following these initial publications, multiple institutions and investigators have reported their experience with robotic hysterectomy or comparisons among robotic hysterectomy, laparoscopic hysterectomy, and abdominal hysterectomy. These experiences will be listed in detail later. To date, no randomized trials have compared these techniques, although a multi-institutional, international trial to compare abdominal radical hysterectomy to laparoscopic or robotic radical hysterectomy for early-stage cervical cancer has been proposed by Obermair.
Hysterectomy for Benign Indications
Following the initial feasibility studies noted previously, a 2007 study by Kho and associates reported the initial experience with 91 patients undergoing robotic-assisted hysterectomy with or without removal of adnexa, appendix, and lysis of adhesions. Estimated blood loss was 78 mL, mean operating time was 127 minutes, average length of stay was 1.35 days, and a complication rate of 8% was reported. A study by Matthews and another by Landeen compared robotic hysterectomy to laparoscopic hysterectomy and open hysterectomy. Reports by Payne, Shashoua, Nezhat, and Giep have compared robotic hysterectomy to laparoscopic hysterectomy.
Table 17.1 summarizes several published reports of robotic hysterectomy for benign indications. In general, robotic hysterectomy was associated with a longer operating time, lower blood loss, similar to shorter length of stay, and similar complication rates when compared to laparoscopic hysterectomy. When compared to abdominal hysterectomy, surgery times were longer, blood loss was lower, length of stay was shorter, and complication rates were lower for patients treated with robotic hysterectomy. It should be noted that all of these studies were retrospective in design. Open hysterectomy and laparoscopic hysterectomy used for comparison were performed prior to the introduction of robotic hysterectomy.
In a study of 256 patients undergoing robotic hysterectomy with uteri weighing 250 to 3,020 g, with the median weight at 453 g, Payne et al. reported median operating times of 167 minutes for women with uteri weighing 500 g or greater, compared to 126 minutes for women with uteri weighing less than 500 g. Blood loss increased as uterine size increased, hospital length of stay was 1 day, and a major complication rate of 2% was reported.
Surgical Technique of Robotic Hysterectomy