Renal Blood and Plasma Flow
Low rates of fetal and neonatal renal blood flow (RBF) exist in a variety of animal species, whether normalized to body weight, surface area, or kidney weight. RBF is mainly determined by a combination of cardiac output (CO) and more importantly the degree of renal vascular resistance (RVR). RBF in the human fetus, estimated by Doppler ultrasonography, increases from 20 mL/min at 25 weeks of GA to more than 60 mL/min by 40 weeks of GA and reaches adult levels by 2 years of age (
25,
26). Developmental changes in both CO and glomerular vascular resistance contribute to this postnatal increase in RBF. For instance, the previable fetus receives about 5% of the CO, while the 1-week-old term infant receives about 9% and the adult kidney receives between 20% and 25% of the total CO (
27). Given that nephrogenesis is complete well before final levels of RBF are achieved, the maturational increase in RBF cannot be completely explained by increases in renal mass.
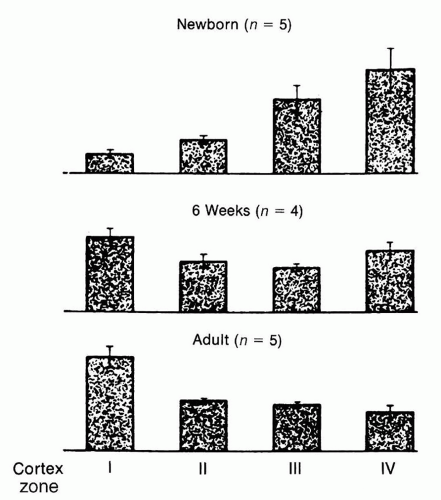
Assessment of intrarenal RBF distribution in the fetal kidney shows significant differences compared to the adult kidney, reflecting differences in the relative size, number, and maturity of glomeruli present in the different regions of the kidney during development. While the predominance of blood flow in early fetal life, as expected, is distributed primarily to the medulla and inner cortex, renal maturation is accompanied by a redistribution of blood flow toward the superficial or outer cortex (
28,
29,
30,
31) (
Fig. 39.1). Thus, at maturity, about 93% of RBF goes to the cortex
(which constitutes about 75% of the renal mass), whereas only 7% is distributed to the renal medulla and perirenal fat.
The maturational increase in RBF results more from the change in intrarenal distribution and the decrease in RVR (
31) than from the rise in CO (
32,
33,
34). The RVR, localized both at the afferent and efferent arterioles, is much higher in the newborn than in the adult (
32). Interestingly, the postnatal fall in RVR occurs at a time when the systemic vascular resistance increases about sixfold (
32). Data suggest that increases in vasodilatory humoral factors, such as nitric oxide, in combination with simultaneous decrease of the vasoconstrictive RAAS mediate, at least in part, the developmental reduction in RVR. Ultimately, the balance of afferent and efferent arteriolar resistances determines not only the RVR and RBF but also the hydrostatic pressure within the glomerulus and level of glomerular filtration rate (GFR).
Anatomic factors also contribute to the developmental increase and redistribution of RBF. For instance, the complexity of the glomerular capillary network varies early in postnatal life. Inner cortical glomeruli at this age generally have a smaller number of capillaries compared to adults, although they appear similar in overall structure. In addition, few efferent arterioles have vasa recta that descend into the medulla, and thus, most connect directly to the venous system resulting in arteriovenous shunting (
33).
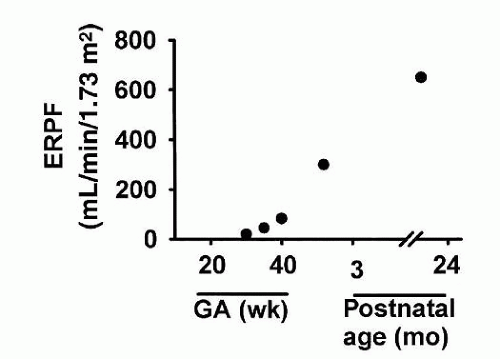
Effective renal plasma flow (ERPF) has traditionally been calculated from the renal clearance of the organic acid para-aminohippurate (PAH). PAH predominately enters the renal tubule through secretory mechanisms within the S2 segment of the proximal convoluted tubule (PCT). Renal plasma flow (RPF) increases rapidly between 30 and 40 weeks of GA, reaching adult values by 1 to 2 years of life (
26) (
Fig. 39.2). PAH clearance averages 150 mL/min/1.73 m
2 body surface area (BSA) in full-term infants at 2 weeks of life, increasing to almost 200 mL/min/1.73 m
2 by 3 months of age (
34). Published values of RPF in premature infants must be interpreted with caution because acid secretory pathways are immature during this time and few nephrons have vasa recta allowing for appropriate delivery of PAH to the basolateral surface of PCT cells.
Renin-Angiotensin-Aldosterone System
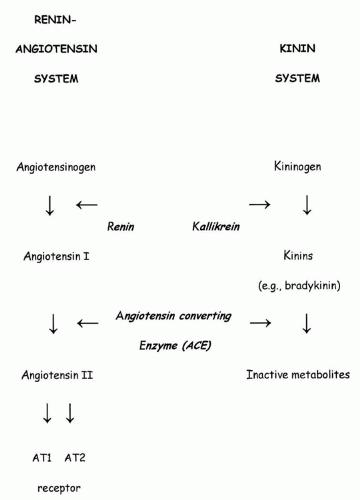
The RAAS, pivotally involved in blood pressure (BP) regulation as well as in sodium and water homeostasis, is very active in the fetus and newborn (
35,
36,
37) (
Fig. 39.3). Plasma renin activity (PRA) is inversely related to GA in the fetus and newborn, decreasing from 60 ng/mL/h at 30 weeks of gestation to about 10 to 20 ng/mL/h by term (
38). Although there is a significant decrease in PRA
in utero, studies demonstrate that PRA at term is three to five times higher than are adult levels (
39,
40,
41). Similar to adults, PRA in the fetus increases with volume depletion or hypoxia and decreases with fluid excess or &bgr;-adrenergic inhibition. As expected, the high levels of renin in neonates are associated with elevated circulating levels of AII and aldosterone that generally exceed those measured in the adult (
42,
43,
44). These high levels may reflect either increased rates of overall secretion or simply low metabolic clearance rates relative to body size. The effect of AII on glomerular hemodynamics depends on relative activation of AT1R and AT2R, which mediate, respectively, vasoconstriction and vasodilatation of the efferent arteriole (
45).
Prostaglandins
Prostaglandins (PGs), particularly PGE2 and PGI2 (prostacyclin), synthesized by the endothelial cells of both the afferent and efferent arterioles, help buffer against circulating vasoconstrictive agents and thus maintain effective RBF and GFR. Urinary excretion of PGs is high in the fetus (
46), presumably reflecting a high rate of renal synthesis. Urinary excretion of PGE2 and prostacyclin metabolites in the premature infant are 5 times that noted at term and 20 times that measured in older children (
47).
PG synthesis from arachidonic acid is mediated by the enzyme cyclooxygenase (COX), which is the inhibitory target of various nonsteroidal anti-inflammatory drugs (NSAIDs) (
48). Two isoforms of COX have been identified, each representing a different gene product and subject to differential regulation. COX-1 has been proposed to participate in glomerulogenesis (
49), whereas COX-2 regulates renal perfusion and glomerular hemodynamics (
50). Differences in intrarenal COX-1 and 2 localization between the adult and fetal human kidney may account for the variable renal responses to PG inhibition observed between the two groups (
49).
Maternal administration of indomethacin, a nonselective COX inhibitor, increases fetal RVR and reduces fetal RBF, GFR, and urine output, ultimately leading to oligohydramnios (
51,
52,
53). Postnatal administration of a PG synthase inhibitor to preterm infants (
54), to close a patent ductus arteriosus (PDA), may also compromise renal function, leading to a reduction in RBF, GFR, and urine volume.
Renal Nerves and the Adrenergic System
The renal vascular bed of the fetal kidney is less reactive to renal nerve stimulation than is that of the newborn and adult kidney (
55). In contrast, circulating catecholamine levels, particularly norepinephrine, are very high just before and immediately after birth (
56) and fall to adult values within a few days of life. The high plasma levels of catecholamines act directly to increase afferent arteriolar tone and indirectly, via stimulation of renin and AII release, to increase efferent resistance, possibly contributing to the maintenance of the high RVR characteristic of the neonatal kidney (
57). The fetal and neonatal kidney demonstrates enhanced sensitivity to catecholamines compared to the adult kidney, related in part to developmental differences in adrenergic receptor density (
58).
Dopamine and Dopamine Receptors
Under normal conditions in adults, dopamine has a biphasic response in the renal vasculature. Low concentrations of dopamine, through binding to dopaminergic receptors, lead to marked vasodilation and increased RBF, whereas high concentrations, via their effect on &agr;-adrenergic receptors, result in vasoconstriction. In contrast, fetal and neonatal kidneys have a blunted response to low-dose dopamine (
59), which results from a limited generation of the vasodilatory second messenger cyclic adenosine monophosphate (cAMP) (
60) and a low density of renal dopamine-1-like receptors (
61). In contrast, intrarenal dopamine infusions in both fetal and neonatal animals (
62) lead to marked vasoconstriction, given the abundance of &agr;-adrenoceptors present by term (
63).
Atrial Natriuretic Factor
Atrial natriuretic factor (ANF) release from atrial cardiocytes is stimulated in the fetus by an increase in intracardiac pressure and atrial distention (
67,
68,
69), and levels fall in response to a decrease in central venous pressure, such as during hemorrhage (
70). ANF has multiple functions within the mature kidney such as antagonizing renal vasoconstriction, increasing GFR, and inhibiting renin secretion, ultimately promoting tubular sodium excretion (
71). However, the natriuretic and diuretic response to systemically infused ANF in the newborn is attenuated compared to adults (
72,
73,
74). While ANF receptors have been identified on near-term fetal glomerular membranes, ANF binding capacity is age dependent, increasing sevenfold between fetal and adult life (
75). The blunted response of the immature subject to ANF can additionally reflect an ineffective production of the second messenger cyclic GMP (
73).
Glomerular Filtration
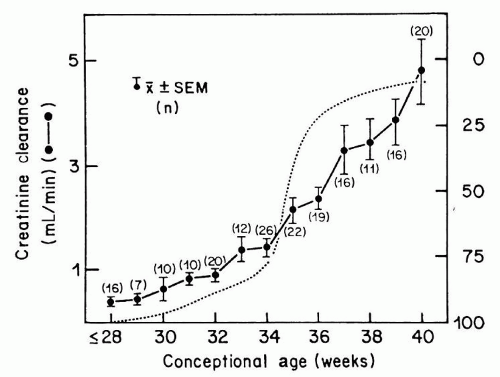
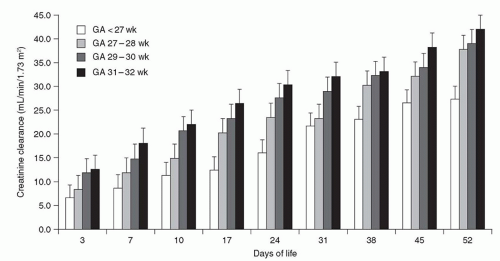
The first glomerulus is detected as early as 9 weeks of gestation, and GFR in the human fetus begins immediately thereafter (
76). Estimates of GFR correlate well with postmenstrual age (PMA), a relationship that persists whether the fetus remains
in utero or is born prematurely (
77,
78). Specifically, GFR averages approximately 8 to 10 mL/min/1.73 m
2 at 28 weeks and increases to 25 mL/min/1.73 m
2 by 34 weeks of PMA. After 34 weeks of GA, the GFR often increases by three- to fourfold within 1 week (
77,
79), coinciding with completion of nephrogenesis (
Fig. 39.4). Thus, an infant born prematurely at 28 weeks of GA shows little increase in GFR until the infant is about 6 weeks old, that is, until a PMA of 34 weeks is attained and nephrogenesis has been completed (
80). Of note, GFR continues to increase rapidly during the first 4 months of life, followed by a slower rise to adult levels by 2 years of age (
25,
81,
82).
At birth, the more mature glomeruli in the juxtamedullary cortex are nearly as large as are glomeruli in the adult kidney. As such, deep glomeruli have higher filtration rates than do the more recently formed superficial glomeruli, which may not begin filtration for some time. Single nephron GFR (SNGFR) depends on four factors: mean glomerular transcapillary hydraulic pressure difference, plasma oncotic pressure, glomerular plasma flow rate, and glomerular capillary ultrafiltration coefficient, which depends on surface area. Studies suggest that the rise in overall GFR is mainly due to an increase in SNGFR of superficial nephrons through an increase in glomerular surface area and in glomerular hydrostatic pressure related to enhanced perfusion of the renal cortex (
28,
33,
83) (
Fig. 39.1).
Autoregulation of Renal Blood Flow and Glomerular Filtration Rate
Autoregulation in the adult kidney allows for maintenance of constant RBF and GFR even as the mean arterial pressure (MAP) and renal perfusion pressure vary widely (typically, 80 to 150 mm Hg). Autoregulation is accomplished primarily by changes in the RVR at the level of the afferent and efferent arterioles. Although MAP (i.e., 20 to 60 mm Hg) in the fetus and neonate is less than the lower limit of autoregulatory range defined for adults, experimental evidence suggests that the fetus and newborn are able to autoregulate RBF appropriately in the setting of their low arterial pressure (
84,
85,
86).
The autoregulatory response to a decrease in MAP is mainly due to a combination of renal afferent arteriole dilation with subsequent constriction of the efferent arteriole. The latter effect is due, at least in part, to enhanced renal sympathetic tone, renin release, AII generation, activation of the AT1R (
45,
87), and activation of hormones such as AVP and endothelin, which enhances proximal tubular sodium and water reabsorption.
Tubular Handling of Electrolytes
The axial and polarized (apical vs. basolateral) distribution of transport proteins along sequential segments of the nephron allows the kidney to reabsorb the bulk of glomerular filtrate proximally and then, in more distal segments, adjust the solute and water content of the urine to maintain homeostasis. Overall, the fully differentiated kidney is generally a reabsorptive organ when it comes to sodium, bicarbonate, phosphate, amino acids, and glucose. Potassium, on the other hand, is both reabsorbed and secreted while hydrogen ions are predominately secreted to help maintain metabolic balance. Thus, the kidney of the full-term, but not necessarily preterm, infant is uniquely suited to meet its developmental stagespecific metabolic demands.
Sodium
Full-term infants are in a state of positive sodium balance, a requisite for appropriate somatic growth. Although the sodium intake per unit of BSA is generally smaller in the newborn than in the adult, the magnitude of this positive balance remains relatively constant within a wide range of sodium intake (
92). This positive sodium balance is achieved predominately through enhanced tubular reabsorption of sodium rather than a low GFR (
93). Unfortunately, the tendency of the full-term neonatal kidney to retain significant amounts of filtered sodium may become problematic under conditions of salt loading. For instance, full-term newborn infants when given a sodium load in excess of 12 mEq/kg/d experience a rise in serum sodium levels, abnormal increase in weight, and generalized edema (
94). The fractional excretion of sodium (FENa) is the ratio of filtered Na that is excreted in the urine, expressed as a percent. FENa in the full-term newborn generally averages about 0.2% (
95). Furthermore, after the first few hours of postnatal life, urinary sodium excretion declines rapidly, possibly secondary to contraction of the extracellular fluid (ECF) volume (
96).
In contrast, FENa may be as high as 20% during early fetal life, and then decreases progressively during gestation (
95,
96,
97). Premature infants of less than 30 weeks of GA show elevated values of FENa, which may exceed 5% (
95,
98,
99). These infants have urinary sodium losses exceeding dietary sodium intake, even with formula designed for preterm infants or with fortified breast milk, and are at risk for a negative sodium balance (i.e., hyponatremia of prematurity) and loss of body weight. They may require, after the first few postnatal days, at least 2 (and some up to 10) mEq/kg/d of supplemental sodium to maintain a normal serum sodium concentration and remain in positive balance (
100). Interestingly, one small randomized trial suggested that sodium supplementation in preterm infants may, in fact, improve neurodevelopment (
101).
Sodium is freely filtered at the glomerulus. The initial two-thirds of the proximal tubule of the suckling rat reabsorb approximately 50% of the filtered load of sodium and water (
93,
102,
103), values only slightly less than those reported in the adult (
102,
104). Studies in several mammalian species demonstrate increases in the reabsorptive capacity of the proximal tubule after birth, consistent with maintenance of glomerulotubular balance during postnatal development (
90,
105). Premature infants, thus, represent a state of functional imbalance in glomerulotubular feedback whereby the reabsorptive sodium capacity of the proximal tubule lags behind increases in GFR (
106,
107).
The fractional reabsorption of sodium along the loop of Henle increases by about 20% during postnatal development (
102), consistent with functional maturation of this nephron segment. Sodium is absorbed in the thick ascending limb of the loop of Henle (TALH) through the furosemide- and bumetanide-sensitive Na-K-2Cl tritransporter located in the urinary membrane and is extruded from the cell at the basolateral membrane by the Na-K-ATPase pump. In contrast to the maturational increase in sodium reabsorption in the loop of Henle, the fractional reabsorption of sodium along the more distal segments is greater in younger than older animals, thereby explaining the sodium retention and blunted response to sodium loading characteristic of the young animal (
93,
108).
Clearance studies in preterm infants (
100,
102,
103,
104,
106,
107,
109,
110,
111) suggest that the percent of filtered sodium reabsorbed by the proximal tubule increases by about 5% between 28 and 34 weeks of GA, whereas the percent of distal sodium reabsorption increases by more than 15% during this same period. However, because the proximal tubule reabsorbs a large percentage of the filtered load of sodium, the small percentage increase in fractional reabsorption in this segment contributes to the postnatal increase in renal sodium retention as much as does the larger percentage increase in the distal tubule.
Distal sodium reabsorption occurs in the cortical collecting duct (CCD) by apical sodium entry into principal cells through the amiloride-sensitive epithelial sodium channel (ENaC) and its extrusion at the basolateral membrane by the Na-K-ATPase. In the fully differentiated nephron, the cellular effects of aldosterone induce increases in the density of apical ENaC channels and stimulation of Na-K-ATPase activity (
111). The net effect of these actions is enhanced sodium absorption. Although high levels of aldosterone prevail through early postnatal life (
45,
112), clearance studies in premature infants (
45,
113) and investigations in neonatal laboratory animals (
114) reveal a blunted responsiveness of the immature kidney to aldosterone. The density of aldosterone-binding sites, receptor affinity, and degree of nuclear binding of hormone receptor appear to be similar in immature and mature rats (
28), suggesting that the early hyposensitivity to aldosterone represents a postreceptor phenomenon. The resulting relative hypoaldosteronism in the premature infant results in an inability to conserve sodium, manifested clinically by weight loss and hyponatremia. In addition, the sodium wasting, characteristic of the preterm infant, may also be a result of a paucity of ENaC in the urinary membrane of the distal nephron during this time (
115).
Urinary sodium excretion during maturation is regulated by the RAAS, renal sympathetic innervation, ANF, dopamine, and glucocorticoids. Direct stimulation of renal nerves in fetal and newborn sheep lead to sodium retention (
116), a response qualitatively similar to that observed in adult animals and attributed to norepinephrine acting on &agr;-adrenergic receptors (
117). In contrast, studies in newborns indicate a relatively poor natriuretic response to ANF (
72,
118) as well as dopamine (
113,
114,
119,
120,
121,
122) compared to their adult counterparts. Circulating levels of glucocorticoids, including cortisol and corticosterone, surge in many species during or just
before the period of weaning (
122,
123). Both endogenous gluco-and mineralocorticoids bind to the mineralocorticoid receptor with equal affinity (
124). Although blood glucocorticoid concentrations are approximately 100-fold higher than are aldosterone concentrations, the metabolism of cortisol into inactive derivatives by 11&bgr;-hydroxysteroid dehydrogenase type 2 (11-&bgr;-HSD2) within the CCD protects the mineralocorticoid receptor from glucocorticoids (
124). The presence of ENaC, mineralocorticoid receptor, and low levels of 11-&bgr;-HSD2 (in the CCD) suggest that glucocorticoids may act as sodium-retaining steroids during early postnatal life (
125).
Potassium
Potassium is transported actively across the placenta from mother to fetus (
126), and thus, fetal potassium is maintained at levels exceeding 5 mEq/L even in the face of maternal potassium deficiency (
126,
127). Unlike adults, who are in net zero balance, growing infants maintain a state of positive potassium balance (
128,
129). The relative conservation of potassium early in life is associated with higher plasma potassium values as compared to adults (
102,
107,
129,
130). These levels average 5.2 mEq/L from birth to age 4 months, decreasing to 4.2 mEq/L by 3 years of age (
130). Under normal circumstances, potassium retention by the newborn kidney is appropriate and a requirement for growth.
Potassium is freely filtered at the glomerulus. Approximately 50% of the filtered potassium is reabsorbed along the proximal tubule in both newborns and adults (
102). Up to 40% of the filtered load of potassium reaches the superficial distal tubule of the newborn, in contrast to about 10% in mature animals, providing evidence for functional immaturity of the loop of Henle (
102,
131). Urinary potassium excretion is derived almost entirely from secretion in distal segments of the nephron, including the CCD. In the adult nephron, potassium secretion occurs by the principal cells of the CCD in association with the electrochemical reabsorption of sodium ions through apical ENaC. The low rates of potassium excretion characteristic of the newborn kidney are due, at least in part, to a low potassium secretory capacity of this segment (
132) given reduced delivery of tubular sodium (in full-term infants) in the setting of low dietary Na intake. Furthermore, an increase in tubular fluid flow rate does not stimulate potassium secretion in the neonatal CCD of the rabbit, as it does in the fully differentiated segment, until after weaning (
133,
134). Baseline and flow-stimulated potassium secretion appear to be limited early in life by a paucity of small-conductance (SK) (
135) and calcium-activated maxi-K channels (
134), respectively, in the urinary membrane of the CCD. The developmental expression of immunodetectable renal outer medullary potassium (ROMK) channels, the molecular correlate of the SK channel (
136,
137), and, shortly thereafter, the maxi-K channel immediately precedes the appearance of baseline and flow-stimulated potassium secretion, respectively, in the CCD. In general, the limited potassium secretory capacity of the immature kidney becomes clinically relevant particularly under conditions of potassium excess.
Calcium
A state of positive calcium balance, characteristic of growing individuals, is sustained by the coordinated interaction of bone, intestine, and kidney. Calcium represents the most abundant mineral in the body and plays a diverse role as a major constituent of bone and teeth, as well as in neuromuscular activity and intracellular signal transduction. Urinary excretion of calcium is inversely related to GA and varies directly with both urine flow and sodium excretion (
138). High rates of calcium excretion may contribute in part to early neonatal hypocalcemia, which is commonly seen in the first 24 to 48 hours of life (
139). The urinary calcium-to-creatinine ratio in full-term infants ranges up to 1.2 mg/mg during the first week of life but may exceed 2 mg/mg in premature neonates (
138,
140). In children more than 2 years of age, the ratio decreases to approximately 0.2 mg/mg, which persists through adult life (
141). The high fractional excretion of calcium in preterm infants may be related to maturational changes in the tubular handling of calcium.
Approximately 50% of filtered calcium is reabsorbed along the superficial proximal tubule in mature rats, yet only 1% of filtered calcium is excreted (
102), suggesting that, in the adult, a large portion of filtered calcium is also reabsorbed at a site beyond the proximal tubule or in deep nephrons (
102). The fractional reabsorption of calcium in the loop of Henle, like that for sodium, potassium, and chloride, is low in newborn rats, increasing significantly with advancing postnatal age (
102,
142). Furosemide, through its effect on inhibiting the apical TALH tri-transporter leading to loss of the luminal positive charge, increases urinary calcium excretion resulting in an enhanced risk of promoting NC and nephrolithiasis. Absorption in both the proximal tubule and TALH is predominantly coupled to sodium absorption and is a passive process through paracellular means. Of interest, calcium absorption in the TALH occurs through tight junctions containing paracellin-1, which when mutated leads to familial syndromes such as hypomagnesemic hypercalciuria and NC (
143). In contrast, calcium reabsorption within the distal nephron is active, is transcellular, and is regulated independently of sodium (
144).
The principal hormones that regulate renal calcium excretion in the adult are parathyroid hormone (PTH), 1,25-dihydroxyvitamin D, and calcitonin (
145). Under normal conditions, a reduction in serum calcium results in the release of PTH from the parathyroid glands. PTH directly leads to enhanced calcium concentrations by direct effects on the nephron, and indirectly through the PTH-induced synthesis of the active vitamin D metabolite 1,25-dihydroxyvitamin D, which stimulates intestinal calcium absorption. In mature animals and adults, PTH decreases urinary calcium excretion by stimulating calcium reabsorption across the cortical TALH and distal convoluted tubule (
146,
147,
148,
149). Although PTH-responsive adenylate cyclase has been found in preterm rabbits (
150) as well as preterm and full-term newborns (
151,
152), the administration of exogenous PTH has minimal effect on renal calcium or phosphorus handling (
153). Thus, it has been suggested that neonatal hypocalcemia may be a result of end-organ unresponsiveness to PTH. Of note, renal production of 1,25-dihydroxyvitamin D increases rapidly after birth, provided that the concentration of the substrate, 25-hydroxyvitamin D, is adequate (
154).
Systemic calcium homeostasis is controlled, in large part, by the extracellular G protein-coupled calcium-sensing receptor (CaSR), located on parathyroid and kidney cells in which it senses the extracellular calcium concentration and in turn alters the rate of PTH secretion and renal calcium reabsorption in the TALH and early distal nephron (
155,
156). There is little expression of CaSR in the fetal kidney (
157) but steady-state abundance of CaSR mRNA and protein increase significantly during the first week of life (
157).
Phosphate
Inorganic phosphate (Pi) is critical for appropriate growth and development given that it is a major component of bone, muscle, and membrane phospholipids as well as critical for many cellular processes that involve adenosine triphosphate (ATP). Thus, it is imperative that neonates and older infants maintain a higher serum phosphate concentration than do adults. The plasma Pi concentration in infants is 4.5 to 9.3 mg/dL and decreases to 3.0 to 4.5 mg/dL in adulthood (
158). This is achieved through enhanced phosphate reabsorption by the kidneys early in life, which progressively declines with advancing age (
159,
160). The fractional reabsorption of phosphate increases from 85% of the filtered load at 28 weeks of GA to almost 99% at term, decreasing thereafter to approximately 85% between 3 and 20 months of age (
161). The high renal reabsorptive capacity for phosphate early in life allows the infant to retain a large portion of phosphate absorbed from the gut and sustain a state of net positive phosphate balance (
161).
Ninety percent of plasma Pi is freely filtered at the glomeruli with 10% being protein bound. The movement of Pi across the apical brush border membrane of the PCT is the rate-limiting step in tubular Pi reabsorption. Pi entry in the cells of the PCT is coupled to sodium and is dependent on the electrochemical gradient delivered by the basolateral Na/K ATPase pump (
162). While three Na
+-Pi cotransporters have been described to date, the expression of the Na
+-Pi cotransporter type II (NaPi2) is highly influenced by dietary Pi intake and hormones such as PTH and growth hormone. Studies have also demonstrated that NaPi2 expression is significantly greater in juvenile animals, under normal conditions, and decreases with advancing age (
163). Furthermore, the phosphaturic effect of PTH is blunted early in life despite the normal circulating PTH levels in the immediate postnatal period, a response mediated, in large part, by the presence of growth hormone (
164) preventing the PTH-induced internalization of apical Na
+-Pi cotransporters in the PCT.
It was initially believed that a low GFR in fetal life was responsible for the limited urinary Pi excretion, but studies in experimental animals demonstrated an overall enhanced renal tubular reabsorption of phosphate early in life. The fractional reabsorption of phosphate in the newborn proximal tubule and distal nephron is higher than is that in the adult (
165,
166). The high intrinsic rate of phosphate reabsorption measured in neonatal proximal tubules has been attributed to an abundance of a growth-related sodium phosphate cotransporter protein in the luminal membrane (
167), a high membrane fluidity of the immature nephron that increases the transport activity of the Na
+-Pi cotransporter (
168), a low intracellular phosphate concentration (
169), and the favorable hormonal milieu prevailing in the perinatal period (
170,
171). Nephron heterogeneity may also explain, in part, the limited urinary phosphate excretion observed in the rapidly growing animal. Because deep nephrons reabsorb more phosphate than do cortical nephrons (
171,
172), and nephrogenesis begins in the juxtamedullary region, the kidney of the immature animal may contain a relatively greater number of functioning nephrons with a high capacity for phosphate reabsorption.
In contrast, the renal tubule of the preterm infant has limited ability to reabsorb phosphate. The tubular reabsorption of phosphate (at normal serum phosphate levels) is 56% in preterm infants born at 23 to 25 weeks of GA and increases to 85% at 26 to 31 weeks of GA and reaches almost 90% at full term (
161). Thus, high phosphaturia in preterm infants may result in a net negative phosphate balance and osteopenia of prematurity if sufficient phosphate intake is not provided. Importantly, in the absence of supplemental phosphate, the serum phosphate level decreases, and once the serum level is below the renal threshold, renal phosphate reabsorption may reach 99% (
173).
Magnesium
Ninety-seven percent of the filtered magnesium is reabsorbed by the mature nephron (
174), largely a function of the TALH. Magnesium reabsorption is regulated by a number of hormones, including PTH, calcitonin, glucagon, and AVP (
175,
176). Additionally, dietary magnesium restriction or loading stimulates or inhibits magnesium reabsorption, respectively; this response is mediated by the CaSR in the cortical TALH and distal tubule (
177,
178). Micropuncture analysis shows that the proximal tubule of the adult animal reabsorbs only about 10% of the filtered magnesium, whereas that of the young developing rat reabsorbs about 60% of the filtered load (
102). Thus, postnatal maturation is associated with a decrease in the fractional reabsorption of magnesium in the proximal tubule (
102). Overall, the avid retention of magnesium within the PCT by the immature kidney likely contributes to the elevated plasma magnesium levels noted during early postnatal life (
179). From a clinical perspective, administration of loop diuretics such as furosemide inhibits magnesium absorption, similar to that seen with calcium, and increases magnesium excretion as a result of the inhibition of the apical tri-transporter and modification of the transepithelial voltage within the TALH (
180).
Amino Acids
The renal reabsorption of many amino acids, including threonine, serine, proline, glycine, and alanine, is lower in newborn animals and humans than in adults, often resulting in aminoaciduria (
187,
188). This does not appear to be a generalized defect in amino acid reabsorption, because other filtered amino acids (e.g., methionine, isoleucine, leucine, tyrosine) are reabsorbed more completely. Specific transporters for acidic, basic, and neutral amino acids have been identified in the urinary membrane of proximal tubules in newborn kidneys (
189,
190,
191,
192). The transient limitation in net transtubular reabsorption of amino acids characteristic of the neonate may arise from intrinsic differences in activity and transport capacity of these discrete transport systems, and/or a lower rate of amino acid efflux out of the cell into the peritubular circulation in the neonate compared to the adult, a mechanism that also would account for the high intracellular concentrations of amino acids observed early in life (
189).
Acid-Base
The acid-base status of the fetus is maintained by both placental and maternal mechanisms. The fetal kidney in the second half of pregnancy, however, is able to acidify the urine (
193,
194). Immediately after birth, the acid-base state of the full-term newborn is characterized by a metabolic acidosis (
195) with respiratory compensation generally occurring within 24 hours following birth (
196). The normal range for serum bicarbonate is lower for preterm infants (16 to 20 mmol/L) and full-term infants (19 to 21 mmol/L) than for children and adults (22 to 28 mmol/L). The lower levels of buffer base concentration in the blood of infants can be accounted for, in part, by the inability to completely excrete the by-products of growth and metabolism (
197).
The concentration of bicarbonate in plasma is determined predominately by the renal bicarbonate threshold, which is lower in preterm and term infants than adults (
198,
199,
200). The low bicarbonate threshold characteristic of the newborn is considered to reflect nephron heterogeneity and/or a low fractional reabsorption of bicarbonate in the immature kidney (
128). In the adult nephron, proximal tubular bicarbonate reabsorption is mediated by the presence of an apical sodium-hydrogen exchanger (NHE) and carbonic anhydrase (which facilitates the interconversion of carbonic acid to water and carbon dioxide). Experimental evidence suggests that the low neonatal bicarbonate reabsorption is a product of low activity of carbonic anhydrase as compared to mature kidneys (
201,
202), even though carbonic anhydrase activity is detected in early fetal kidney development (
203,
204). Postnatal maturation of the proximal tubular capacity for bicarbonate reabsorption has been proposed to be a result of increases in the activity of both the NHE and carbonic anhydrase within this segment (
205,
206,
207).
The renal response to acid loading increases with advancing gestational and postnatal ages. When compared to adult subjects given a comparable acid load, the infant exhibits a larger fall in blood pH and bicarbonate concentration, a smaller and slower fall in urine pH, and much smaller increase in urinary titratable acid and ammonium excretion (
208,
209). Premature infants born at 34 to 36 weeks of GA exhibit rates of net acid excretion and ammonium generation that are about 50% lower compared to term infants. Thus, net acid excretion increases to levels observed in term newborns only after 3 to 4 weeks of age (
209). In response to acid loading with ammonium chloride, urinary pH values of less than 6 are rarely observed in premature infants until the second month of life (
210). In contrast, by the end of the second postnatal week, urinary pH values of 5 or lower are consistently observed in term infants (
211,
212). The rates of ammonia synthesis and excretion are low in the neonate (
213) and, in response to acid loading, do not increase to mature values until 2 months of age (
200,
211,
214). Of note, phosphate loading, administration of cow milk (which is rich in protein and phosphate) instead of breast milk, or high-protein feeding enhances the ability of the newborn to excrete titratable acids and ammonia (
212,
215).
The final site of urinary acidification is the renal collecting duct. Functional immaturity of this segment and particularly the acid-base transporting intercalated cells therein may further limit the ability of the neonate to effectively eliminate an acid load (
216,
217). Postnatal differentiation of intercalated cells has been shown to include changes in the morphology and function of these specialized cells with an increase in density along the CCD.
Urinary Concentration and Dilution
The fetal metanephric kidney produces large amounts of hypotonic urine that contribute significantly to the volume and composition of amniotic fluid (
94,
218,
219). Urine osmolality early in life is typically one-fifth that achieved by the adult (
65). Yet, the fetal nephron is able to concentrate urine under conditions of stress, such as that induced by maternal water deprivation (
220), hemorrhage (
213), or infusion of AVP (
221,
222). After fluid deprivation for 12 to 24 hours, the maximal urine osmolality achieved in premature and full-term newborns is 600 and 800 mOsm/kg, respectively (
223,
224). The kidney’s maximal urinary concentrating ability (approximately 1,000 to 1,200 mOsm/kg) in both children and adults is generally not attained until at least 6 to 12 months of age (
223,
225).
Urinary concentration requires a corticomedullary osmotic gradient, the pituitary release of AVP, and the ability of the collecting duct principal cells to increase its water permeability in response to AVP. The limited urinary concentrating ability of the infant appears to be due primarily to an inability to generate a corticomedullary osmotic gradient and diminished responsiveness of the distal nephron to AVP (
225,
226).
The capacity to concentrate urine has been directly related to elongation of the loops of Henle and their penetration into the medulla (
227). The inner medulla and renal papillae are poorly developed in the immature kidney. In the rat, the 1.6-fold increase in length of the renal medulla correlates well with the 1.5-fold increase in urine osmolality observed between 10 and 20 days of age (
227). In addition to anatomic maturation of the loops of Henle, urinary concentration requires the generation of a high interstitial solute concentration gradient in the medulla, which is underdeveloped early in life (
223,
228). Generation of the corticomedullary osmotic gradient necessitates the postnatal maturation of several processes involved in urinary concentration including sodium chloride reabsorption by the TALH, urea sequestration by the principal cells of the inner medullary collecting duct (in the presence of ADH), and functional activation of aldose reductase, an enzyme necessary for generation of intracellular osmolytes, important for maintenance of cell function in the concentrated milieu (
229,
230). Furthermore, the functionally limited countercurrent multiplier system in the immature kidney prevents the appropriate buildup and maintenance of a medullary gradient needed for effective urinary concentration.
In contrast to urinary concentrating abilities, premature infants less than 35 weeks of GA, studied under conditions of maximal water diuresis, can decrease their urine osmolality to 70 mOsm/kg, whereas infants greater than 35 weeks of GA are able to reduce urine osmolality to 50 mOsm/kg (
106). Although the proximal tubular sodium reabsorption is relatively less mature in the preterm infant compared to adults, the high avidity of the distal nephron for sodium reabsorption allows the neonate to generate a free water clearance greater than that in adults (
92,
231,
232). Yet, despite the high capacity for free water clearance, the ability of the neonate to excrete a hypotonic load is limited, presumably as a result of the low GFR.
Antidiuretic Hormone
The limited ability of the immature kidney to concentrate urine is not a result of an inability to synthesize and secrete ADH. In fact, circulating levels of ADH are elevated in preterm and term infants and decrease rapidly in term infants within 24 hours of birth (
63,
233). Studies in fetal and newborn animals (
65,
234,
235), and in human infants (
233,
236), indicate a qualitatively appropriate response to osmolar or volume stimuli known to affect ADH release. Furthermore, exogenous administration of ADH or 1-desamino-8-d-AVP (DDAVP) to healthy 1- to 3-week-old newborns leads to a response, albeit of shorter duration and reduced magnitude than that observed at 4 to 6 weeks (
237). Cumulative evidence suggests that the blunted sensitivity of the fetal and neonatal kidney to AVP and limited concentrating ability of the neonatal animal is not a result of a paucity of V2 receptors (V2R; receptor to which ADH binds in the collecting duct) (
238,
239), aquaporin channels involved in water transport across renal tubule epithelia (
240), or efficiency of coupling to second messengers (adenylate cyclase and protein kinase A activity) (
241,
242,
243) after the first week of postnatal life but is limited primarily by the poorly developed corticomedullary osmotic gradient.