Diaper Dermatitis
Diaper dermatitis is common in newborns. It is characterized by a pink to red scaly rash localized to the diaper area and is caused by prolonged exposure to urine and stool combined with the frictional forces of the diaper. This irritant contact dermatitis should be distinguished from numerous other dermatologic conditions affecting the diaper area. Irritant contact dermatitis affects skin contacting the diaper, such as the pubis, penis, labia majora, scrotum, upper thighs, and buttocks. Generally, the creases are spared. Continued irritant exposure may produce painful fissures and ulcerations (Jacquet erosive dermatitis), especially in premature infants (e-
Fig. 51.1). Involved skin is susceptible to secondary infection, primarily with
Candida albicans,
Staphylococcus aureus, and
Streptococcus pyogenes.
C. albicans causes pustules on a red base (“beefy-red”) that, after rupture, form erosions surrounded by fine superficial white scale. Frequently, the inguinal and gluteal folds are involved.
S. aureus and
S. pyogenes cause a superficial skin
infection called impetigo, which is characterized by pustules on a red base that form yellow crusts when ruptured. Certain types of
S. aureus produce an epidermolytic toxin, causing superficial bullae. However, secondary bacterial infection with
S. aureus and
S. pyogenes may be characterized primarily by superficial erosions and crusting. Streptococcal infection is malodorous, which may be used to distinguish it from
Candida infection.
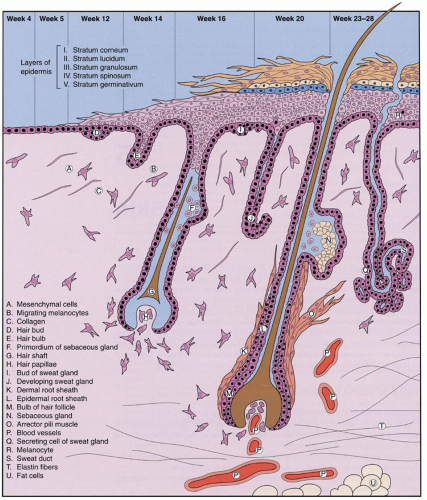
Factors involved in the genesis of diaper dermatitis include increased skin wetness (e.g., sweat, urine, and stool) of the stratum corneum (
14), which makes the skin more susceptible to friction from the diaper material, and irritation from elevated pH caused by urinary ammonia and from activation of fecal proteases and lipases in the alkaline milieu (
15,
16). When alkaline urine combines with feces, the potential for irritation is compounded. Breastfed infants have been noted to have stools with lower pH, which may account for their decreased incidence of diaper dermatitis.
The differential diagnosis of irritant diaper dermatitis includes psoriasis, seborrheic dermatitis, secondary syphilis, histiocytosis, acrodermatitis enteropathica (AE), and cystic fibrosis. In those conditions, however, unlike diaper dermatitis, skin outside the diaper area is usually involved.
Treatment of diaper dermatitis should be targeted toward reducing skin wetness, minimizing contact of the skin with urine and feces, and eradicating infectious organisms. Skin wetness and irritation can be minimized in several ways. Ultra-absorbent diapers have been shown to be superior to cloth diapers in decreasing skin wetness and maintaining an acidic skin pH (
17,
18). Frequent diaper changes also help to minimize wetness. Barrier ointments (e.g., containing petrolatum) help keep urine and feces from contacting the skin. Other commercial products containing zinc and vitamins A and D also may be effective. The skin should be gently cleansed with water or, if necessary, a mild nonalkaline soap before barrier ointment reapplication. Complete removal of barrier ointment with diaper changes is not necessary and, if attempted, may further exacerbate skin injury. When diaper dermatitis is unusually severe or recalcitrant or when risk factors are likely to be ongoing (e.g., malabsorption syndromes), a thick layer of pectin-based paste without alcohol, followed by a barrier ointment or zinc oxide ointment, may prove efficacious. Cholestyramine compounded in Aquaphor helps neutralize bile acids and is effective in erosive dermatitis (
19). Commercially available diaper wipes can exacerbate irritation and should be reserved for healthy-appearing skin or for circumstances in which soap and water are not available.
Mild, low-potency topical corticosteroids such as 1% hydrocortisone ointment can be applied if inflammation is significant; however, higher-potency topical steroids should be avoided because of the risk of cutaneous atrophy, striae, adrenal suppression, and Cushing syndrome. Combination antifungal-steroid compounds such as Lotrisone and Mycolog II have no use in treating neonatal skin conditions involving the diaper area. Each contains a potent topical corticosteroid (betamethasone dipropionate, and triamcinolone 0.1%, respectively) that when placed under diaper occlusion can cause cutaneous and systemic side effects. Use of powders also should be avoided. Fungal or bacterial superinfection can be managed with appropriate topical antimicrobial agents (
20). For more severe bacterial and fungal infections, oral agents may be considered. Topical antifungal agents such as nystatin, clotrimazole, miconazole, or ketoconazole can be safely applied to newborn skin. Bacterial infection generally responds to mupirocin or bacitracin. Neomycin carries a higher risk of allergic contact sensitization and should be avoided.
Seborrheic Dermatitis
Seborrheic dermatitis is characterized by red skin with white-yellow waxy scale, occurring on the scalp, on the eyebrows, and in the intertriginous areas, especially in the posterior auricular, neck, axillary, and inguinal folds. Typical scale is absent in the intertriginous areas. The eruption begins at 2 to 3 weeks of age and in some infants becomes widespread over the ensuing months. The vast majority of patients with seborrheic dermatitis improve significantly during the first year of life. Persistent and severe seborrheic dermatitis may be associated with human immunodeficiency virus (HIV) infection and other underlying systemic diseases.
Alterations in fatty acid metabolism, nutrition, and/or immunity, and infection with Pityrosporum ovale have been thought to contribute to seborrheic dermatitis, but no firm cause has been established.
Atopic dermatitis, psoriasis, Langerhans cell histiocytosis (LCH), scabies, and dermatophyte infections are the principal conditions that must be excluded. In general, atopic dermatitis produces itching and psoriasis is more difficult to control than is seborrheic dermatitis. Sometimes, several visits with careful observation and assessment of the therapeutic response are required to establish the proper diagnosis; skin biopsy sometimes becomes necessary for diagnosis.
When infants have limited involvement, no specific therapy is required other than mild shampoo and/or mineral oil to gently remove the scale. For more adherent, thick scalp scale, warm olive oil covered with a warm moist towel assists in removing scale. Care
must be taken not to remove hair, when removing scale, since permanent alopecia can occur with repeated hair removal. Selenium sulfide shampoo or 2% ketoconazole shampoo may be helpful but should be kept out of the eyes. Ketoconazole 2% cream, hydrocortisone 0.5% to 1% (lotion, cream, ointment), pimecrolimus cream, and tacrolimus ointment applied once to twice daily are alternative therapies.
Psoriasis
Psoriasis can produce various cutaneous patterns such as guttate (teardrop), papules, pustules, and plaques. Most infants with psoriasis develop thick, pink plaques with firmly adherent thick white (micaceous) scale in a distribution similar to seborrheic dermatitis. Psoriasis in the newborn period is uncommon, and congenital psoriasis is rare. When newborns are affected, they tend to have localized plaques, on the scalp, diaper area, or hands. One can also see isolated nail and nail-fold involvement. Rarely, newborns develop generalized pustular erythroderma with fever.
The cause of psoriasis is unknown, but genetic influences play an important role because children with two psoriatic parents have an approximately 50% lifetime risk (
21). Infection (
S. pyogenes), cold weather, emotional stress, medications (beta-blockers, antimalarials), and withdrawal from systemic corticosteroids have all been shown to provoke psoriatic flares. Psoriasis improves with therapies targeting T lymphocytes and cytokines produced with T-cell activation (
22).
A family history of psoriasis and “soft signs” of psoriasis (e.g., pink skin in the gluteal cleft, nail pitting, geographic tongue, and rash in the umbilicus) are useful aids to diagnoses.
Medium-strength topical corticosteroids, tacrolimus, and pimecrolimus are the mainstay of therapy for infantile psoriasis. Short-contact therapy (application for <30 minutes, followed by washing) with anthralin is a safe therapy, but may be limiting because of skin irritation. Calcipotriol may be applied, but to limited areas because of risk of vitamin D toxicity. Tar ointments are effective but have an unpleasant odor, and stain the skin, and may not be well accepted by caregivers. Biologic treatments targeting T lymphocytes and associated cytokines improve psoriasis in adults, but have not been studied in infants.
Atopic Dermatitis
Newborns with atopic dermatitis develop red, itchy papules and plaques involving the forehead, cheeks, and flexural surfaces, with relative sparing of the diaper area; there may be widespread generalized redness and scaling. Well-defined criteria exist to diagnose atopic dermatitis (
23). In the newborn, atopic dermatitis is recognized when an infant has the typical rash and a family history of atopy, asthma, or atopic dermatitis. In the young infant, pruritus may not be evident because of lack of scratching. Many infants are colonized with
S. aureus, and all are susceptible to viral infections (e.g., herpes simplex virus [HSV], smallpox virus, molluscum contagiosum virus, and human papillomavirus).
Although up to 30% of infants with atopic dermatitis have concurrent food hypersensitivity, food allergy does not cause atopic dermatitis. Rather, atopic dermatitis is a complex disorder subject to genetic, immunologic, and environmental influences (
23). Atopic dermatitis must be differentiated from seborrhea, psoriasis, fungal infection, and scabies, and the treatment is similar to other noninfectious inflammatory conditions. Moisturizers, low-potency topical steroids, tacrolimus, pimecrolimus, avoidance of cutaneous irritation, and treatment of secondary infections are the fundamental elements of therapy.
Erythroderma
Erythroderma is a generalized red eruption as a result of a number of inherited and acquired conditions. Inflammatory conditions (atopic dermatitis, seborrheic dermatitis, mastocytosis), infectious conditions (syphilis, herpes, S. aureus), metabolic conditions (methylmalonic aciduria, maple syrup urine disease, cobalamin deficiency), genetic skin diseases (ichthyosis, Netherton syndrome, nonbullous ichthyosiform erythroderma), and immunodeficiency (severe combined immunodeficiency, common variable hypogammaglobulinemia) are examples of conditions that can result in erythroderma. These infants have a compromised epidermal permeability barrier and are at risk for hypothermia, dehydration, and sepsis. Diagnostic evaluation should be directed by history and physical examination. Typically, a skin biopsy is not helpful during acute erythroderma. Management focuses on fluid and temperature homeostasis, utilizing intravenous fluids and emollients, and placing infants in isolettes with warm humidified air. The caloric requirements of the infant are dramatically increased, and parenteral nutrition is sometimes required. Emollients applied two to three times daily assist in healing the skin barrier. Fissures should be treated with topical antibiotics such as mupirocin or bacitracin. Infants with signs of skin infection (e.g., crusting, oozing, malodorous areas) and sepsis should be cultured and treated with appropriate systemic antibiotics.