Disorder |
Salient Gross Features |
Salient Microscopic Features |
Comment |
Twin birth with MoDi placenta |
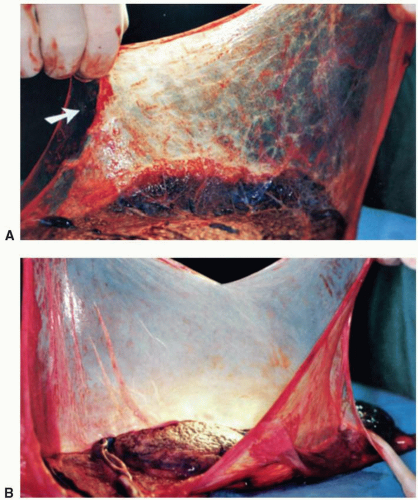
Single placental disk, two amniotic sacs, of same or dissimilar size, and dividing septus attached to the fetal surface are seen; velamentous or marginal insertion and a single umbilical artery are more frequent; vascular anastomoses are present in 85% to 100% of cases; pallor of the donor and congestion of the recipient territory are seen when there is twin transfusion syndrome; amnion nodosum may be present in the donor territory. |
Histologic findings reflecting the gross findings are seen; the dividing septum in composed of two amnions only, without intervening chorion. |
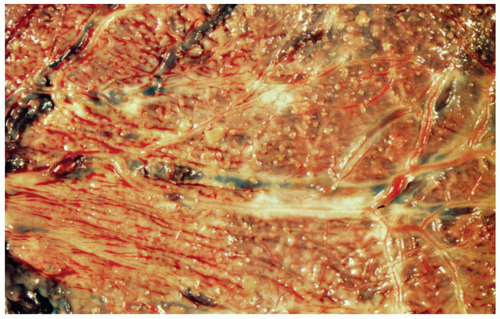
Superficial vascular anastomoses can be visualized by naked eye examination; injection studies may be done to confirm these findings; injection studies are the only way to demonstrate deeper arteriovenous anastomoses via a shared lobule. The twin transfusion syndrome is seen in 15% to 30% of twins with MoDi placenta. |
Twin birth with DiDi placenta |
Two placental disks may be completely separate or fused, resembling the MoDi placenta; the fusion may be partial, with fusion of only a portion of membranes; a dividing septum is present in fused placentas. Vascular anastomoses are extremely rare. |
Dividing septum shows chorion between the two amnions. |
Twin transfusion is extremely rare; the pathology of separate DiDi placentas is same as that of singleton placenta.a |
Twin birth with MoMo placenta |
Only one amniotic sac and a single placement disk amniotic fold representing disrupted septum of a previously DiDi placenta may be present; vascular anastomoses are common; findings of twin transfusion syndrome described above may be seen; the cords often become entangled. |
No specific features other than vascular anastomoses. |
Demonstration of vascular anastomoses should be done when twin transfusion syndrome is done when twin transfusion syndrome is present. This is the rarest type of twin placement with a high incidence of fetal morbidity |
Vanishing twins |
Plaques of previllous fibrin, embryonic remnant on the membranes, and a second amniotic sac, with or without an embryo may be found; the embryonic remnant appears as a flattened yellow plaque, with or without ocular pigment. |
The embryonic remnant shows autolyzed embryonic tissues. |
There are few reports describing the pathologic features of the placenta; the placenta should be carefully examined for detection of the embryonic remnant; the twin may be lost via vaginal bleeding. |
Fetus papyraceous/fetus compressus (FP/FC) |
FP/FC representing a dead twin is identifiable as a plaque of dehydrated remnant, with or without identifiable fetal parts; umbilical cord torsion or massive infarction may cause fetal death. |
Histologic findings reflect the gross abnormalities; autolyzed fetal tissues are seen in the sections from FP/FC. |
Careful gross examination is essential to detect FP/FC, roentgenograms may be taken to show the skeleton of FP/FC; the causes of fetal death may not be evident. |
Acardiac twin |
MoMo placenta with artery-to-artery and vein-to-vein anastomoses between the viable twin and acardiac twin placental territories. |
No specific microscopic features other than vascular anastomoses |
Vascular anastomoses should be sought; the acardiac twin has no heart or a severely malformed heart; other malformations may also be present. |
Intrauterine growth restriction (IUGR) |
IUGR is associated with maternal factors (preeclampsia, chronic renal disease, substance abuse, etc.), fetal factors (severe congential anomalies chromosomal disorders, intrauterine infarctions, etc.), and placental factors (extrachorial placenta, velamentous insertion of cord, maternal floor infarctions, VUE, extensive infarction or perivillous fibrin deposition, etc.); placenta findings related to the maternal, fetal, and placental factors are seen. |
Histologic findings related to fetal, maternal and placental factors are seen. |
The placenta is small, which may be a reflection rather than a cause of IUGR; the cause of IUGR may not be evident may be normal except for its size. |
Erythroblastosis fetalis |
Enlarged weight ans size, pallor, intervillous thrombi |
Villi; immature with persistent cytotrophoblast, numerous normoblasts in capillaries, villous edema, hemosiderin in chorionic macrophages |
Severity of placental changes is related to severity of fetal anemia; the fetus is hydropic |
Nonimmunologic hydrops fetalis (NIHF) |
The causes of NIHF include genetic and metabolic disorders, chromosomal abnormalities, cardiac and pulmonary anomalies, thalassemia, fetomaternal hemorrhage, fetal infection, fetal tumors, arrhythmias congenital nephritic syndrome; placental findings related to the disorders are present. |
Histologic findings related to the associated condition are seen. |
In approximately 22% of NIHF cases, no associated condition can be found. |
Chromosomal disorders (trisomy 13, 18, 21) |
Small placenta, high incidence of single umbilical artery |
Villi: delayed maturation, hypovascularity, large, atypical Hofbauer or trophoblastic cells. |
Karyotyping may be done on the chorion or amnion. |
Metabolic disorders |
Large hydropic placenta |
Vacuoles in the syncytiotrophoblasts, intermediate trophoblast, Hofbauer cells, endothelium and fetal WBCs in the villous capillaries. |
The vacuoles represent the accumulated metabolite, which is dissolved during processing; electron microscopy may give clues regarding the precise diagnosis (e.g., Niemann-Pick disease glycogenosis type IV); biochemical study of snap-frozen fresh placental tissue is essential for definitive demonstration of enzyme deficiency. |
Antepartum stillbirth, intrauterine fetal death (IUFD) |
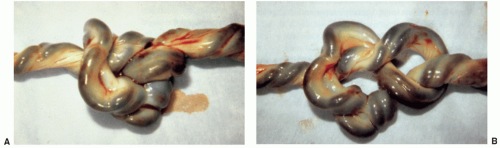
Massive infarction retroplacental hematoma, large chorangioma, true knot, torsion or stricture of the cord, nuchal cord, intrauterine infection, maternal floor infarction, and extensive perivillous fibrin deposition are placental lesions than may lead to IUFD. |
Histologic findings reflecting these placental lesions are seen; assessment of the interval between IUFD and delivery can be made on the basis of histologic findings. |
Maternal conditions (e.g., preeclampsia) and fetal conditions (e.g., erythroblastosia fetalis) may also cause IUFD; certain placental abnormalities such as stromal fibrosis, hypovascularity thrombosis of villous edema are secondary to IUFD; confined placental mosaicism may occur in rare cases. |
a There may still be significant fetal-to-placental transfusion after delivery of the first-born twin, but true twin-to-twin transfusion is so unlikely as to necessitate looking for a different etiology should this be a consideration. |
MoDi, monochorionic diamnionic; DiDi, dichorionic diamnionic; MoMo, monochorionic, monoamnionic. |
From Joshi VV. Handbook of placental pathology. New York: Igaku-Shoin, 1994, with permission. |