Stabilization in the Intensive Care Unit following Surgery
Following the operation, the ICU service should obtain a standardized handoff from the anesthesiologist and the surgeon (
68). Included in this handoff are details about the anesthetic regimen, the operative findings and surgical procedure performed, as well as duration of CPB, aortic cross-clamp, and circulatory arrest (if applicable). If performed, the results of the transesophageal echocardiogram and any pressure or CO-oximetry measurements should be communicated. Information regarding vascular access, pacing wires, chest drains, and intraoperative arrhythmias or other complications should be discussed.
Invasive hemodynamic monitoring is used in nearly all neonates and infants following cardiac surgery. One or more central venous lines are typically placed in the operating room. Sites for line placement are chosen depending upon the patient’s anatomy, anticipated postoperative course, and clinician preference. Some clinicians prefer to avoid placement of central venous lines in the subclavian and jugular veins in patients with single-ventricle physiology due to concerns for thrombosis of the upper extremity systemic veins. Intracardiac lines may be inserted by the surgeon prior to chest closure through the right atrial appendage to the right atrium (RA line) or through the right upper pulmonary vein or left atrial appendage to the left atrium (LA line).
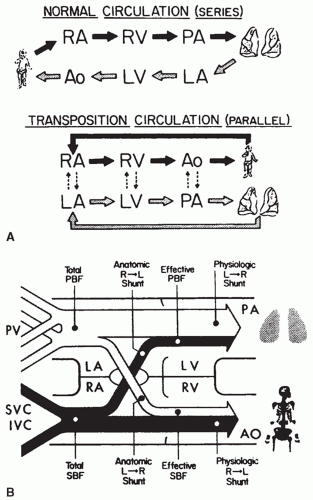
A pulmonary artery catheter may be placed through an internal jugular or subclavian vein, the right atrium, or right ventricular outflow tract. These catheters are infrequently used in the current era but may be informative in selected patients at high risk for postoperative pulmonary hypertension, residual VSDs, or residual right ventricular outflow tract obstruction. Continuous monitoring of pulmonary artery pressure provides precise knowledge of the severity of pulmonary hypertension and immediate feedback as to the effectiveness of interventions to lower pulmonary artery pressure. Significant lability in pulmonary artery pressures during suctioning of the endotracheal tube or awakening from sedation may be a sign that a patient is not ready to be weaned. Measurement of a step-up in oxygen saturation from a superior vena cava or right atrial catheter to a pulmonary artery catheter may be helpful for the detection of significant residual left-to-right shunting (
69). A pullback pressure tracing from the pulmonary artery to the right ventricle may be obtained at the time of removal of the pulmonary artery catheter, which quantifies any residual gradient across the right ventricular outflow tract. Some pulmonary artery catheters also have a thermistor tip, thus allowing cardiac output to be calculated by the thermodilution technique.
Proper interpretation of intracardiac and vascular pressure measurements (markers of ventricular loading conditions) is beneficial for the detection of residual lesions, the titration of volume administration, and the implementation of interventions that modify vascular tone. Interpretation of the atrial waveforms may provide insight into the presence of significant atrioventricular valve regurgitation or rhythm disturbances.
An arterial line facilitates continuous blood pressure monitoring and frequent arterial blood gas sampling. Care should be taken to ensure that blood pressure measurements are accurate. Dampened waveforms or pressures measured distal to stenotic arteries may give the false impression of hypotension. For example, arm blood pressure measurements in a patient who has, or had in the past, an ipsilateral Blalock-Taussig shunt may be diminished due to subclavian arterial stenosis or occlusion. The waveform and pulse pressure may be informative as the cardiac pathophysiology. For example, significant diastolic runoff may produce a wide pulse pressure in the presence of a systemic-to-pulmonary shunt, aortopulmonary collateral arteries, or severe aortic regurgitation. A narrow pulse pressure, along with tachycardia and hypotension, may signify cardiac tamponade. Patients who underwent repair of coarctation or aortic arch reconstruction should have four-extremity blood pressure measurements taken to document any residual aortic arch gradient.
A variety of factors may contribute to erroneous data obtained from invasive monitoring, including inappropriate transducer
height, and bubbles or clots in the catheters. Information obtained from invasive monitoring cannot be used in isolation but, when placed in the context of the overall clinical picture, can be very useful to guide management in the early postoperative period.
Complications associated with central lines are uncommon but include air embolus, thrombus, infection, bleeding, and arrhythmias (
70). When using LA lines in patients with two-ventricular repairs, and with any central line in those with single-ventricle physiology, care must be taken not to inject air into the systemic circulation. Complications at the time of intracardiac catheter removal include retention and bleeding; the latter has been shown to occur more commonly with pulmonary artery catheters (
71,
72). Consideration should be given to coagulation status and surgical availability when removing intracardiac lines and pulmonary artery catheters.
Assessment of the heart rhythm is an important part of the initial evaluation following surgery. The heart rate and rhythm should be continuously monitored at the bedside, and these data should be reviewable on a telemetry system. An ECG is usually obtained in the immediate postoperative period to serve as a new baseline should the patient subsequently develop a tachyarrhythmia or myocardial ischemia. Atrioventricular synchrony is important for optimizing cardiac output. Temporary pacing wires may be placed before chest closure in the operating room. These pacing wires may be interrogated when attempting to clarify arrhythmia mechanism. They also may be used to pace-terminate certain tachyarrhythmias and are effective for pacing in the setting of junctional ectopic tachycardia (JET), heart block, or other bradyarrhythmias. Sensing and capture thresholds should be assessed regularly. These wires are quite safe and may be removed at the bedside when no longer clinically indicated.
Temperature should be monitored and regulated closely. High temperature increases metabolic demands and may adversely affect hemodynamics and neurodevelopmental outcomes, whereas hypothermia may increase systemic vascular resistance and cause bradycardia.
A directed physical examination should be performed to assess the cardiopulmonary status and adequacy of the surgical repair. Any murmurs or gallops should be noted, although dressings and chest tubes may limit the auscultatory findings. It is common to hear a friction rub in the first few days following cardiac surgery, usually due to accumulation of a small amount of fluid in the pericardial space. The liver span should be noted. Adequate chest rise and breath sounds should be noted bilaterally. The quality and symmetry of peripheral pulses and perfusion of the extremities are useful means of assessing the adequacy of the systemic circulation. Caution must be used when attempting to estimate the adequacy of cardiac output by assessing capillary refill or peripheral-core temperature gradients, as both have a poor correlation with cardiac index, systemic vascular resistance index, and lactate levels (
73).
Chest tubes should be assessed for location and proper function. In infants, the tubes may generally be removed when drainage falls to less than 20 to 30 mL/d and when there is no evidence for chylothorax or air leak. A CXR should be obtained upon admission to the ICU and for the first few days after surgery, as critically ill infants have a high percentage of films with an abnormality requiring intervention (
74). Particular attention should be given to the location of all tubes and lines, as well as the heart size and lung fields.
The surgeon will occasionally leave the chest “open” after a Norwood procedure and other complex neonatal operations, with the skin closed using a Silastic patch, until hemodynamic stability can be achieved, bleeding controlled, and myocardial edema can decrease (
75,
76). The risk for mediastinitis may be increased when the chest is left open, and prophylactic antibiotics are typically continued during this time period (
75). Delayed sternal closure may be performed in a few days in the operating room or in the ICU. Of note, much higher doses of narcotics are required during sternal closure when compared to most other procedures performed in the ICU. When the sternum is closed, respiratory compliance may decrease, necessitating additional ventilatory support.
Cardiopulmonary interactions play an important role in the physiology of neonates and infants following cardiac surgery (
77). Arterial oxygen saturation is monitored continuously by pulse oximetry. Arterial blood gas analyses are obtained frequently, and attention should be given to ensure adequate oxygenation and ventilation for the individual patient’s physiology. Manipulations of PaCO
2, PaO
2, pH, and mean airway pressure may be used in the context of the patient’s physiology to modulate hemodynamics. Mechanical ventilation and sedation are also useful to minimize oxygen consumption in patients with limited cardiopulmonary reserve. Respiratory acidosis may increase pulmonary vascular resistance, and efforts should be made in most cases to avoid it (
78). Low functional residual capacity may predispose patients to atelectasis and increased pulmonary vascular resistance, whereas pulmonary overdistension may increase pulmonary vascular resistance and decrease cardiac output. More generous tidal volumes are often needed after CPB when compared to those typically used in patients receiving mechanical ventilation for parenchymal lung disease.
Although early extubation policies have been reported for older infants and children, most neonates and young infants receive at least 12 to 24 hours of mechanical ventilation following congenital heart surgery. Criteria for extubation following cardiac surgery in neonates and infants are similar to those used in other patient populations. These include the presence of adequate cardiac output, appropriate neurologic status to maintain the airway, muscular strength to support respiratory pump function, acceptable gas exchange, and the absence of significant arrhythmias, bleeding, or fever.
Standard laboratory values need to be assessed in the early postoperative period. Electrolytes, including magnesium and ionized calcium levels, are monitored and corrected as needed. A complete blood count is initially obtained daily, and hemoglobin levels are monitored more frequently. In general, a hemoglobin level of 10 to 12 g/dL is appropriate for infants following a two-ventricular repair, and a hemoglobin level of 13 to 15 g/dL is reasonable for infants following a palliative operation with ongoing cyanosis. Relative anemia may place unnecessary workload on the myocardium, and transfusion of erythrocytes will improve oxygen delivery following pediatric cardiac surgery. An assessment of coagulation status (prothrombin and partial thromboplastin times [PTTs] and platelet count) is often obtained soon after CPB and repeated as clinically indicated.
In addition to the physical examination, several clinical parameters may be used to assess the adequacy of cardiac output and oxygen delivery in the immediate postoperative period. The presence of a metabolic acidosis, as quantified by a base deficit or lactate level, suggests inadequate systemic cardiac output and requires investigation. Lactic acidosis develops when inadequate tissue oxygen delivery leads to anaerobic metabolism. Following congenital heart surgery, elevated lactate levels in infants and children upon admission to the ICU are associated with increased morbidity and mortality (
79). Venous oxygen saturation may be measured to estimate cardiac output. Urine output and markers of renal function (blood urea nitrogen and creatinine) provide a good estimate of the systemic cardiac output. Oliguria may be seen for 12 to 24 hours after complex cases, but improvement should occur thereafter in most patients. Infants often require inotropic support following CPB, and low-dose dopamine or milrinone is the initial drug of choice at many centers. Inotropic support is discussed in more detail in the “Low Cardiac Output” section below.
Infants may develop significant fluid retention following CPB, which may impair myocardial, respiratory, and gastrointestinal function. Strategies used to minimize this problem in the operating room, including the use of steroids and ultrafiltration, were discussed earlier. Despite the presence of total body fluid overload,
intravascular volume depletion is common in the first few hours following surgery due in part to capillary leak, and one or more fluid boluses may be required. Diuretics are typically initiated 12 to 24 hours after surgery, either as bolus doses or as continuous infusion. Electrolyte disturbances, particularly hypokalemia, hyponatremia, and a hypochloremic metabolic alkalosis, are commonly encountered as diuresis occurs in the first few days following CPB.
Analgesia is provided for all patients following cardiac surgery. High-dose fentanyl is well tolerated and blunts the stress response in neonates following CPB (
80). Morphine or other narcotics are commonly used in the early postoperative period. Benzodiazepines or dexmedetomidine may be administered for amnesia and sedation. Neuromuscular-blocking agents may be used in selected patients to eliminate ventilator dyssynchrony and minimize oxygen consumption in patients with labile hemodynamics.
Gastrointestinal tract motility is decreased following cardiac surgery. Contributory factors include the inflammatory effects of CPB, anesthesia, fluid retention, narcotics, and (in some cases) high central venous pressures or low cardiac output. If these considerations are anticipated to preclude the initiation of enteral nutrition for several days, then parenteral nutrition may be administered. Histamine-2 receptor antagonists may be administered to minimize the risk of upper gastrointestinal bleeding.