Rectum and Anus
Charles N. Paidas
Marc A. Levitt
Alberto Peña
Department of Surgery, Johns Hopkins University School of Medicine, Johns Hopkins Hospital, Baltimore, Maryland 21287.
Department of Pediatric Surgery, Schneider Children’s Hospital, Long Island Jewish Medical Center, New Hyde Park, New York 11042.
Department of Pediatric Surgery, Schneider Children’s Hospital, Long Island Jewish Medical Center, New Hyde Park, New York 11042.
For more than 100 years, surgeons around the world have been grappling with the morphologic consequences of events involving the caudal end of the embryo that occur between the fourth and eighth weeks of gestation. The anomalies are known as anorectal malformations (ARMs). As the embryologic and genetic sequencing of events continue to unfold, the questions of how and why these caudal defects occur are to be answered. For the time being, the pediatric surgeon is consulted for antenatal counseling, postnatal and perioperative opinions, and definitive operative correction of ARMs. Ultimately, the pediatric surgeon should manage the sequelae of these defects, including constipation, fecal and possibly urinary incontinence, and sexual inadequacies, regardless of how impeccable the initial operative correction. As many females with ARMs have approached childbearing age, the pediatric surgeon is frequently consulted by obstetricians. General surgeons also call on the pediatric surgeon for clarification of anatomy in patients with ARMs.
For centuries, an anal orifice was blindly created by making an incision in the perineum of children with imperforate anus. Many of these children did well, probably because these were defects with an anus located very close to the skin of the perineum (low defects). In contrast, most children with higher defects did not survive. In Paris in 1835, Amussat (1) actually performed the first surgical anoplasty by suturing the wall of the rectum to the skin edges without a colostomy. This procedure became the standard for what we now call a low imperforate anus. Until 1953, a key recommendation for the repairs of high imperforate anus was to stay close to the sacrum to avoid damage to the urinary tract during the dissection. Cadaveric dissections by Stephens (2) led him to use a combined sacral and abdominoperineal approach, whereby he pulled the rectum down as close to the genitourinary tract as possible in an effort to preserve the puborectalis sling. In contrast to pre-1953, preservation of the puborectalis sling became the theme for many subsequent operative approaches to ARMs (3,4,5). As more was learned about ARMs, not only the existence of the puborectalis sling, but also the role (if any) such a muscle might play in bowel continence was questioned.
A posterior sagittal approach to ARMs was introduced in 1982 (6,7). Through a wide posterior sagittal incision and with electrical stimulation of muscle, the surgeon can more correctly identify a spectrum of anorectal anomalies and tailor the reconstruction of the perineum. The posterior sagittal anorectoplasty (PSARP) approach to ARMs hopefully facilitates correlations of the anatomy of these malformations to the clinical results obtained for each type of male and female defect.
EMBRYOLOGY
Since the mid-1990s, the science of embryology has evolved from the mere study of isolated events in development to a two-stage process that links the formation of the body plan with the organs and tissues that belong in the respective regions of this body plan. We now know that there are a set of genes that “pattern” the embryo so organs and tissues appear where they are spatially meant to be and that an additional set of genes are responsible for the actual formation of these organs and tissues (8). As a result of a process called gastrulation, this body plan is set up by the end of the third week of gestation. There is substantial literature identifying the genes responsible for the patterning of the cranial region of the embryo and the tissues these genes specify (9). However, there is a paucity of data describing patterning of the caudal region of the embryo (10,11,12,13,14). What we have learned about caudal region human embryology is that there is an intimate relationship among muscle, bone, and nerves at the caudal end of
the embryo, demanding a clinical management algorithm that considers this embryologic trio.
the embryo, demanding a clinical management algorithm that considers this embryologic trio.
Anomalies of the anorectum have been previously explained on the basis of an arrest of the caudal descent of the urorectal septum toward the cloacal membrane during the fourth week and ending by the eighth week of gestation (15). The urorectal septum is composed of mesoderm. The growth, migration, and differentiation of this mesoderm constitute a critical pathway for normal descent of the urorectal septum, as well as other mesodermally derived tissues in its vicinity. For example, formation of the muscular and skeletal systems also derives from mesoderm. Furthermore, it is quite likely that embryonic induction of a specific developmental pathway in one group of cells (i.e., movement of the urorectal septum) may induce adjacent tissue (i.e., sacral somites) to transform into muscle, bone, and skin. Substances that might alter this induction of organs or disrupt cell–cell interaction following specification by a genetic code include proteins of the extracellular matrix (laminin, fibronectin, and collagen types I and IV) (16,17) and growth factors. Activation of a sequential family of genes or intracellular biochemical changes may further affect the induction of these tissues. Such is the case for expression of sonic hedgehog (Shh) gene and its secreted protein signaling pathway (18). Transcription factors responsive to Shh, such as Gli2 and Gli3, may also be equally important in the genesis of ARMs and normal caudal region embryology (19). Shh signaling is essential for normal development of the caudal region of the embryo in mice. Mutations in the Shh signaling pathway have produced a spectrum of anorectal anomalies similar to those found in humans (20). Thus, in addition to the spectrum of anorectal anomalies that results from dysmorphogenesis of the mesodermally derived urorectal septum, important consideration must be given to the induction of the caudal regional triad of muscle, bone, and nerve in weeks three through eight of gestation (Table 89-1).
In general, ARMs are not related to either the preimplantation or fetal period, but instead are related to the embryonic period of gestation (2 to 8 weeks). During the third week of embryogenesis, the process of gastrulation transforms the bilaminar germ disk into a trilaminar disk by ingress of epiblasts into the hypoblasts in an area called the primitive streak. Three definitive layers of the newly formed trilaminar germ disk are called ectoderm, endoderm, and mesoderm. The trilaminar germ disk forms the basis for the body plan, both cranially and caudally (21,22). Gastrulation then is synonymous with formation of mesoderm, and thus, the body plan. The implication of dysmorphogenesis this early in gestation is that patterning of the embryo is altered. Also, if something abnormal occurs this early in gestation, we should look regionally for other anomalies. There are, however, some malformations that almost never have associated defects (see “Anatomy, Classification, and Description of Defects”). This has clinical relevance because we know that with ARMs there is a 40% to 50% overall incidence of associated malformations (23,24).
TABLE 89-1 Timetable of Embryologic Events Leading to Formation of the Anorectum. | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
At either end of the embryo, the ectoderm and endoderm fuse, excluding the mesoderm in these areas, and give rise to a cranial buccopharyngeal and caudal cloacal membrane. It is believed that the cloacal membrane fuses and canalizes in humans by the eighth week to give rise to the anus and distal urethra. However, formation of the cloacal membrane and an anal opening from the dorsal segment of the cloacal membrane has been identified as early as 10 days postcoitus in mice. Absence of any of the dorsal part of the developing cloaca and its membrane (as opposed to the urorectal septum) seems to give rise to a spectrum of fistulas or communications of the hindgut with the urogenital tract (25,26). These findings lend credence to the notion that the events leading to formation of ARMs can be ascribed to a much earlier time in gestation (i.e., gastrulation) and are not solely the result of the movement of the urorectal septum. Although these early embryologic errors were observed in a mutant mouse strain (Sd mouse), it is quite possible that this early sequence of dysmorphogenesis can also occur in humans (27). Further examination of the Sd mouse reveals that it has an abnormal notochord, resulting in reduced number of caudal vertebrae and abnormal cloaca and caudal gastrointestinal and genitourinary pathology (28). In ethylene-thiourea–treated rat embryos, ARMs were found in association with abnormalities of notochord development in the lumbosacral area and the cloaca, suggesting a relationship between the notochord, cloaca, and ARMs (29,30). These data suggest that there are very early links in the genetic code of the caudal body plan and the set of genes that subsequently form organs.
During the third week of embryogenesis as gastrulation proceeds, the primitive streak regresses caudally. In the caudal region of the embryo, the streak disappears,
giving rise to a mass of mesoderm cells called the caudal eminence. The caudal eminence gives rise to two very intimately related areas, including the caudal somites of the body and the caudal end of the neural tube. Mesoderm inferior to the second sacral level fuses with the more cranial neural tube. The caudal region of the body plan is dependent on the formation, differentiation, and migration of the caudal eminence and its derivatives. This process is complete by 6 weeks of development.
giving rise to a mass of mesoderm cells called the caudal eminence. The caudal eminence gives rise to two very intimately related areas, including the caudal somites of the body and the caudal end of the neural tube. Mesoderm inferior to the second sacral level fuses with the more cranial neural tube. The caudal region of the body plan is dependent on the formation, differentiation, and migration of the caudal eminence and its derivatives. This process is complete by 6 weeks of development.
In the latter part of the third week of embryogenesis, the mesoderm cells on either side of the regressing primitive streak give rise to paraxial, intermediate, and lateral plate mesoderm. Paraxial mesoderm yields axial skeleton, voluntary muscle, and skin dermis. The intermediate mesoderm produces the urinary system and elements of the genital anatomy. The ventral layer of the lateral plate mesoderm gives rise to visceral mesoderm, and the dorsal layer of the lateral plate gives rise to parietal mesoderm, parts of the limbs, and most of the dermis.
Paraxial mesoderm gives rise to somitomeres, all during the third and fourth weeks, starting in the cranial end and terminating caudally. In humans, somitomeres form somites numbering 39 pairs in a cranial–caudal direction. Somites give rise to axial skeleton, vertebral columns, skull bones, voluntary muscles (i.e., levator muscle complex, external sphincter), and dermis (perineal skin) (31). They also give rise to the segmental pattern within the body wall that eventually gives rise to other structures, such as blood vessels and nerves. The five pairs of sacral somites form the sacrum and associated musculature of the pelvis (levator ani group and external sphincter). The three pairs of coccygeal somites form the coccyx and probably contribute to induction of paraxial mesoderm in the perineum.
Somites subdivide into three types of mesoderm: myotomes, dermatomes, and mesenchymal sclerotomes. Sclerotomes (ventral portion of the somite) develop first and differentiate into vertebrae at all levels of the developing embryo. The adhesion molecule, N-cadherin, has been reported to be important for dissolution of the somite and formation of the sclerotome (32). Along the ventral surface of the body, myotomes are called hypomeres. These hypomeres migrate into lateral plate mesoderm, along with their respective dermatome and spinal nerves (33). The muscles of the perineal region differentiate from hypomeres of the sacral and coccygeal regions of the embryo. As expected, dermatomes and spinal nerves also migrate along with the hypomeres, giving rise to the levator ani musculature, external anal sphincter, and voluntary muscles of the external genitalia and their corresponding nerves (34). Thus, in the caudal region of the developing embryo, like in the cranial region of the body plan, organization and migration of these mesodermally derived somites give rise to the triad of muscle, bone, and nerve.
Regionalization of the postgastrulation mesoderm in the area of the sacrum and coccyx is probably regulated by pattern formation. In the case of the anorectum, pattern formation facilitates localization of the urinary tract anterior to the rectum, as well as a series of migrations that culminate in separate genital, urinary, and gastrointestinal tracts. In addition, the correct position of muscle with respect to the bones of the perineum is also probably dependent on pattern formation. This process is believed to be controlled in part by a class of genes called homeobox genes (35,36,37,38,39) and other families of homeotic genes, all of which are genes that regulate the expression of other genes by producing transcriptional factors. The pharyngeal arches in the cranial region of the embryo are a prototype of patterning regulated by homeobox genes (40). The homeobox or Hox gene expression may specify a code for postgastrulation mesoderm differentiation, such as the paraxial mesoderm, and simultaneously all three germ layers surrounding this area (41,42).
During the fourth week, the neural plate folds into the neural tube by a process called neurulation. Special subpopulations of cells that arise from the lateral regions of the developing neural tube are called neural crest cells. These specialized detached cells give rise to a variety of components of the peripheral nervous system that ultimately innervate the developing gut (including the hindgut). In fact, peripheral sensory neurons and parasympathetic and sympathetic peripheral motor neurons are all neural crest derivatives. An important neurologic milestone related to ARMs, during the fourth week of embryogenesis, is the formation of the spinal nerves from sacral levels 2, 3, and 4, which contributes to the peripheral parasympathetic nervous system. In contrast to the failure of migration and thus absence of ganglion cells observed in Hirschsprung’s disease, abnormal matrix, cell-to-cell communication, or an abnormal induction pathway of neural crest development may be the basis for rectosigmoid proprioceptive and motility problems associated with ARMs. In the absence of any gross anatomic abnormality, the clinical manifestations of such a molecular embryologic phenomenon may include postoperative complaints of constipation and incontinence.
The caudal end of the neural plate (flanked by somites) gives rise to the lower end of the spinal cord. Secondary neurulation takes place once the neural plate has closed (primary neurulation) over a period of 7 weeks and is defined as the formation of the spinal cord at the caudal limit or tail bud of the embryo (43). The tail bud contributes to the sacral and coccyx levels of the spinal cord. The caudal eminence also induces both spinal cord and other paraxial mesoderm cells so ultimately this area forms the nervous system of the caudal part of the body. Defects in the caudal eminence give rise to sacral agenesis and the fatal dysmorphogenic caudal regression syndrome of Duhamel (44) (Fig. 89-1). Muscle, bone, and nerve pathology is associated with this lethal condition that may be the result of abnormal gastrulation in a very specific area of the primitive streak. It is possible that defects in the derivatives of the mesodermally derived caudal eminence are caused by
macromolecules of the extracellular matrix (16). In addition, cell surface glycoproteins and cell adhesion molecules (N-CAM) participate in the transformation of the caudal eminence (45). These disturbances may have a multifactorial etiology and include both genetic and environmental causes.
macromolecules of the extracellular matrix (16). In addition, cell surface glycoproteins and cell adhesion molecules (N-CAM) participate in the transformation of the caudal eminence (45). These disturbances may have a multifactorial etiology and include both genetic and environmental causes.
It is widely held, but has never been proved, that between weeks four and six the primitive gut tube at the level of the cloacal membrane gets canalized and partitioned into an anterior urogenital sinus and a posterior anorectum by the cranial caudal growth of a mesodermally derived partition called the urorectal septum (Fig. 89-2). Ultimately, the urorectal septum fuses with the cloacal membrane, and the fusion site is called the perineal body.
Overgrowth of the cloacal membrane prevents the lateral mesoderm from forming the anterior abdominal wall and the cloaca ruptures. If the cloacal membrane ruptures after complete descent of the urorectal septum, exstrophy of the bladder occurs, whereas rupture before descent of the septum yields cloacal exstrophy.
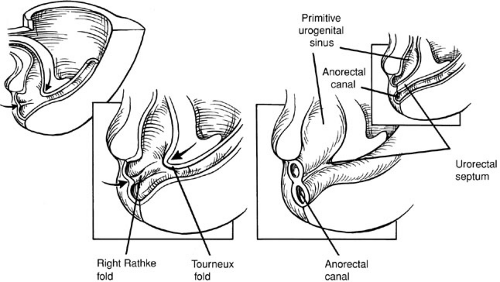
The urorectal septum is a composite of two mesoderm structures: the midline Tourneux fold and two lateral Rathke folds (Fig. 89-2). In contrast to the superior two-thirds of the anal canal, the inferior one-third is derived from ectoderm called the anal pit or proctodeum. The anal membrane resorbs by the eighth week, and this area when fused with the descending mesoderm of the hindgut is called the pectinate or dentate line. Some authors believe that proof of this process of septation of the cloaca or caudal migration of a septum is lacking and the partitioning of the cloaca is the result of normal dorsal–ventral
cloacal development (25,26,46). In fact, in three-dimensional image reconstruction of human embryos, the urogenital sinus and anorectum form early and are separated by the urorectal septum as a passive structure (47). The catenoidal geometric shape of the urorectal septum, like the tracheoesophageal septum, also suggests this is a passive structure (48).
cloacal development (25,26,46). In fact, in three-dimensional image reconstruction of human embryos, the urogenital sinus and anorectum form early and are separated by the urorectal septum as a passive structure (47). The catenoidal geometric shape of the urorectal septum, like the tracheoesophageal septum, also suggests this is a passive structure (48).
The failure of Rathke folds to develop results in arrest of the inferior part of the urorectal septum, and thus, rectourethral (prostatic) fistulas in the male and a common channel (cloaca) for the urethra, vagina, and rectum in the female.
In females, the distal end of the urogenital sinus forms the vestibule and distal third of the vagina. The proximal vagina and uterus are formed following fusion of the paramesonephric ducts (müllerian ducts) and union of these structures with the proximal urogenital sinus. It is believed that formation of the uterus, vagina, and fallopian tubes requires the absence of antimüllerian hormone or müllerian-inhibiting substance (49).
The arrest of Rathke folds usually takes place just below the paramesonephric ducts, but a slightly more caudal failure of Rathke folds could result in a high rectovaginal fistula in the female.
The failure of both Tourneux and Rathke folds could result in rectobladder neck fistulas in both males and females, however, we suspect that this is not true because we have not seen a rectovesical fistula in a female with normal genitalia. Instead, failure of both folds is more likely to be a cloacal anomaly in the female. In the female, failure of formation of both folds can also result in duplicated vaginas and uteruses that empty into one bladder. This concept is consistent with what we find in the spectrum of cloacal anomalies.
Malalignment of Tourneux and Rathke folds may result in formation of a rectourethral (bulbar) fistula in males and a low vaginal or vestibular fistula in females.
Imperforate anus without fistula results from the anal pit not forming. Mesodermally derived Tourneux and Rathke folds are aligned, but the ectodermally derived anal pit has not fused with the mesoderm. Thus, the result is an imperforate anus, but no fistula to the skin.
Formation of the anal pit and failure of the anal membrane to resorb or resorb incompletely results in rectal atresia or anal stenosis, respectively. This fusion defect consists of cloacal ectoderm and descending rectal mesoderm.
Fusion of the genital folds gives rise to a covered anus. This does not happen in females because in the absence of testosterone there is no fusion of the genital folds, but instead they remain separate to form the labia. Defects in the mesoderm at the level of the perineal body where fusion of the ectoderm and endoderm is taking place result in a perineal fistula that by definition is an opening with no surrounding external anal sphincter.
In the next few years, it should not be surprising to find genetic literature linking the formation of the body plan (gastrulation) with postgastrulation organ formation in the caudal region of the embryo. Ultimately, these data should give rise to the regionalized phenomenon of caudal patterning of muscle, bone, and nerve. The Sd mouse and the Shh knockout are excellent models to study ARMs and may be used in the future to probe the two-stage process involving the origin, fate, and interaction of mesoderm (the body plan) and regional organ formation.
GENETICS
As early as 1950, records were compiled about congenital anomalies, their incidence, and their overall contribution to maternal and fetal morbidity and mortality. More than 90% of congenital anomalies develop in the first 8 weeks of gestation and more than one-half of these occur in the first 4 weeks or just after gastrulation (50). As for ARMs, the overall incidence approaches 1 in 5,000 live births (51), and the risk for future pregnancies in a mother of a patient with ARMs is 1 in 100 or 1% (52). Relative to all types of caudal regression anomalies, ARMs are much more common if one separates the incidence of persistent cloaca (1:40,000 to 50,000) and cloacal exstrophy (1:400,000) (53). Yet, we suspect that the real incidence of persistent cloaca is much higher based on the fact that so many alleged rectovaginal fistulas are cloacas. In general, males slightly outnumber females by about three to one, and there seems to be no ethnic predilection.
The genetic basis of ARMs is multifactorial, meaning there is no one single cause (54). ARMs can be caused by a sporadic or an inherited event. Sporadic events are isolated and probably induced by an unknown genetic or environmental cause. It is unclear if a sporadic case is inherited. If, however, another sibling or descendent has an ARM, then one can infer that the malformation is inherited either as an isolated event or as part of a syndrome. The patterns of ARM inheritance include the mendelian disorders (autosomal dominant, recessive, etc.), chromosomal abnormalities (i.e., cat eye syndrome), or environmental teratogens (i.e., maternal diabetes). Some syndromes that include ARMs can be inherited as a result of multiple gene mutations, hence, the term heterogeneous causation. The actual incidence of sporadic versus inherited ARMs is unknown despite literature quotes supporting a higher incidence of isolated ARMs (55). Based on the fact that there is a reported range of associated anomalies between 22% and 72%, it is very possible that sporadic cases are in fact part of a syndrome (24). Granted we have multiple examples of hindgut dysmorphogenesis that can account for these associations, and it is compelling to consider a syndromic etiology as advances in molecular genetics occur. There is some evidence, albeit scant, that isolated low anal
malformations (perineal fistula, covered anus) may be inherited, whereas isolated high ARM lesions are likely to be sporadic embryologic events with little to no risk of familial recurrence (56,57). Similar to the sporadic ARMs, the familial-related ARMs carry a 50% incidence of associated malformations (58).
malformations (perineal fistula, covered anus) may be inherited, whereas isolated high ARM lesions are likely to be sporadic embryologic events with little to no risk of familial recurrence (56,57). Similar to the sporadic ARMs, the familial-related ARMs carry a 50% incidence of associated malformations (58).
Both from a reproductive counseling perspective and for postnatal care, the potential for inheriting an ARM must be considered. Because it is nearly impossible to make an antenatal diagnosis of the more common ARMs, detailed family pedigree may provide a clue to potential hereditary mechanisms. Risks of recurrence within a family can then be based on the pattern of inheritance. For example, if an autosomal dominant condition is identified, it carries a 50% incidence of recurrence, whereas a recessive condition occurs in 25% of offspring. Family genetic counseling about ARMs should also include the potential for association with other system anomalies and chromosomal defects. A prenatal karyotyping should be performed in all cases in which there is a family history of ARMs.
Postnatal physical examination should alert physicians to the possibility that the baby has a syndrome. Table 89-2 is a helpful guide for the extraanal anomalies associated with ARMs. The Mendelian inheritance in man (MIM) number is an online computerized database (59) that can be accessed for a more complete update of each diagnosis. The vertebral, anal, tracheoesophageal, renal, and radial limb anomalies (VATER) (60,61) or vertebral, anal, cardiac, tracheoesophageal, renal, and nonradial limb anomalies (VACTERL) (62,63) associations should not be the sole extraanal associations ruled out when evaluating a patient with an ARM. This is especially important from an embryologic perspective when we consider that the mesoderm involved in the VATER and VACTERL associations as well as all components of the other anomalies listed in Table 89-2 are present by the fifth week of gestation. Likewise, if a baby has skeletal, visceral, or neurologic anomalies, one should look for the constellation of bowel, bladder, or bone anomalies (64).
ANATOMY, CLASSIFICATION, AND DESCRIPTION OF DEFECTS
In general, the frequency of ARMs is slightly higher in males compared with females, and this also includes the potential for associated anomalies. A rectourethral (bulbar and prostatic urethra) fistula is the most frequent defect in newborn males followed by a perineal fistula. Higher defects, such as the rectobladder neck fistula, occur in less than 10% of male series (65,66).
In females, by far the most frequent defect is an imperforate anus with a rectovestibular fistula followed in frequency again by the perineal fistula. Most cases of rectovaginal fistulas are probably misdiagnosed rectovestibular fistulas or cloacas because in Peña’s series of more than 1,600 cases, rectovaginal fistulas were virtually nonexistent. The common cloaca comprises about 10% of the defects in females and ranks third in frequency (65,66).
Imperforate anus without a fistula occurs in less than 5% of cases in both sexes and has a high association with Down syndrome (65,66).
Although there is a long history of proposed classifications for ARMs, the 1984 Wingspread classification (67) of high, intermediate, and low defects has been used extensively (68,69). With respect to therapy and prognosis, however, the classification falls short of implications and utility. A therapy-oriented classification is shown in Table 89-3. This proposed categorization combines diagnosis and treatment, and therefore, should facilitate more homogeneous communication about specific malformations among pediatric surgeons.
Male Defects
Perineal Fistula
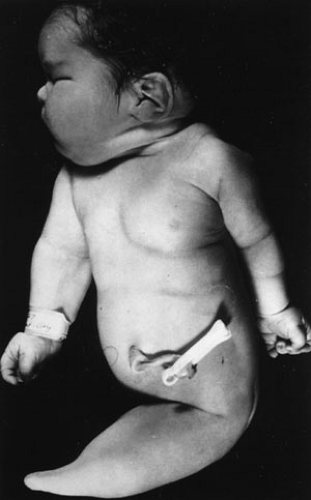
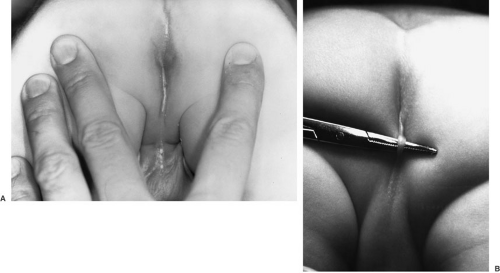
Perineal fistula is a low defect (Fig. 89-3). The lowest part of the rectum opens on the perineum anterior to the center of the external sphincter. The more proximal rectum remains within the muscles of the sphincter. A subepithelial rectoperineal fistula can be found along the midline raphe from the base of the scrotum or penis (Fig. 89-4). Occasionally, a skin tag is encountered (bucket handle deformity) (Fig. 89-4B), below which meconium may be seen coming from the fistulous tract. Male and female patients with a perineal fistula have a well-developed midline groove and anal dimple, normal sacrum, ample sphincter musculature, normal rounded contour of their buttocks, and minimal urinary and neurologically associated anomalies.
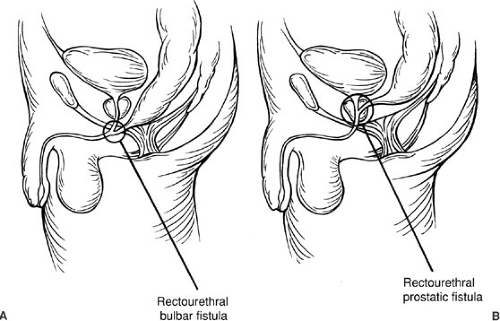
Rectourethral Fistula
The rectourethral fistula defect (Fig. 89-5) is characterized by the rectum communicating with the posterior part of the urethra at its lower (bulbar, Fig. 89-5) or upper segment (prostatic, Fig. 89-5). In general, the patients with a bulbar fistula have a substantial sphincter mechanism, normal sacrum, prominent midline groove, and well-defined anal dimple. In contrast, those males with a rectoprostatic urethral fistula have a more flat contour buttock, poor sphincter mechanism, abnormal sacrum, and poorly defined anal dimple. Most males fit these observations, but there are exceptions such that a rectoprostatic fistula can be associated with a normal sacrum, defined anal dimple, and good muscle.
Above the rectourethral fistula, the rectum and urethra share a common wall. As expected, the common wall is longer in cases of a bulbar fistula in contrast with a prostatic fistula. The rectum in both types of rectourethral
fistulas is usually surrounded by the funnel-shaped voluntary striated muscle mechanism innervated by the sacral plexus (sacral levels 2, 3, and 4).
fistulas is usually surrounded by the funnel-shaped voluntary striated muscle mechanism innervated by the sacral plexus (sacral levels 2, 3, and 4).
TABLE 89-2 Anorectal Malformations (ARMs) in Association with Extraanal Anomalies. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
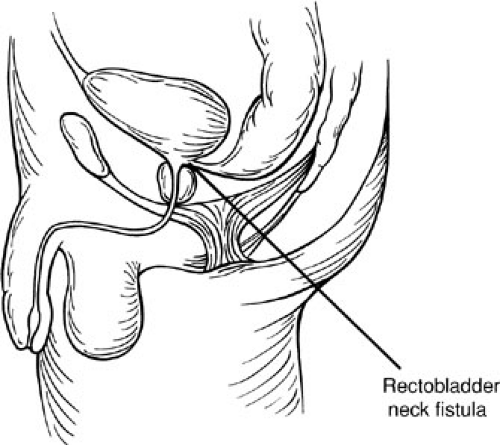
Rectobladder Neck Fistula
In the case of rectobladder neck fistula defect (Fig. 89-6), the rectum opens at the bladder neck in a T configuration. In contrast with a rectourethral fistula common wall, patients with a bladder neck fistula do not share a common wall. In these defects, the rectum is located above the funnel-shaped levator musculature. Ectopic ureters usually open into the bladder close to the site of the fistula. The perineum is usually flat, there is a paucity of perineal muscle, and the sacrum is dystrophic and frequently absent. This ARM has a high frequency of associated anomalies.
Imperforate Anus Without Fistula
In the imperforate anus without fistula defect, the rectum ends approximately 2 cm from the perineal skin. The sphincter mechanism, muscles, and sacrum are usually present, and thus, bowel function is predictably good. Even
though there is no direct communication between the urethra and anus, there is a very thin common wall between these structures.
though there is no direct communication between the urethra and anus, there is a very thin common wall between these structures.
TABLE 89-3 Proposed Classification of Defects. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
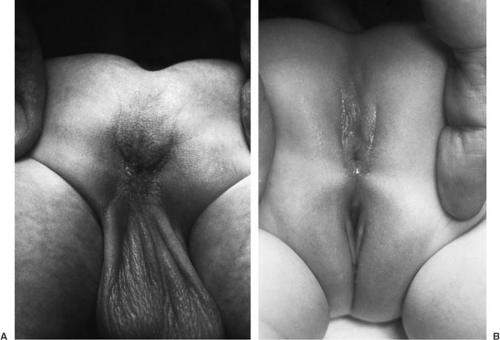 FIGURE 89-3. Perineal fistula in (A) male and (B) female. Electrical stimulation of perineal skin shows that the anal opening is anterior to the external sphincter muscle. |
Rectal Atresia and Stenosis
In the cases of rectal atresia and stenosis, the rectum ends blindly (atresia) or partially communicates with the distal anal canal (stenosis). Typically, these patients have a normal-looking anus that ends blindly 1 to 2 cm above the perineal skin. The atresia or stenosis occurs at the embryologic junction of the anal canal with the rectum. These two structures are separated by a thin membrane or fibrous band. Following repair, continence and sensation are usually excellent. Voluntary muscles, sacrum, and perineum are nearly normal.
Female Defects
Rectoperineal Fistula
The rectoperineal fistula malformation is the equivalent to the low-lying male cutaneous fistula because it is also surrounded by skin. The anus opens onto the perineal body anterior to the external sphincter, yet the opening is posterior to the vestibule of the vagina. These patients have an excellent anal dimple and buttock contour. The sacrum in this defect is normal, as is the presence of the levator musculature. Rectum and vagina are also well separated, and thus, there is no shared common wall.
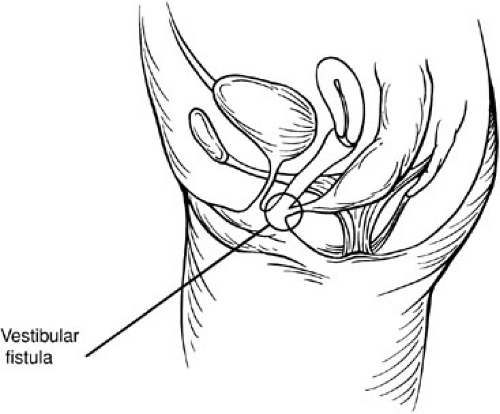
Rectovestibular Fistula
The rectovestibular fistula ARM is characterized by the rectum opening immediately behind the hymen within the vestibule of the vagina (Fig. 89-7). This defect is frequently erroneously diagnosed as a rectovaginal fistula. Just above the fistula, the rectum and vagina are separated by a thin common wall. Yet, these patients usually have excellent musculature, normal sacrum, and anal dimple.
Imperforate Anus Without Fistula and Rectal Atresia and Stenosis
The imperforate anus without fistula and rectal atresia and stenosis malformations has similar anatomic, diagnostic, therapeutic, and prognostic implications in males and females. Imperforate anus without a fistula has a slightly higher incidence in females compared with males.
Persistent Cloaca
The persistent cloaca (Fig. 89-8) defect is characterized by fusion of the rectum, vagina, and urethra into a single
common channel (70). The length of the common channel varies between 1 and 10 cm. A short common channel is usually defined as shorter than 3 cm, whereas common channels longer than 3 cm are considered long-channel malformations. Figure 89-8A is the perineum in a female baby with imperforate anus, small genitalia, and single perineal orifice. Figure 89-8B shows a child with cloaca who has a common channel of approximately 3 cm. In contrast, Figure 89-8C shows a case in which the common channel is much longer. In this situation, separation and mobilization of the three structures, creating two walls out of each common wall between the urethra and the vagina, as well as between the vagina and the rectum, is difficult and most likely requires some form of vaginal replacement and probable laparotomy. Urinary function may be compromised in these long-channel defects. The perineum is usually well developed, and musculature, sacrum, and innervations are adequate in cases of short common channels. Frequently, the vagina in cases of persistent cloaca is distended and full of secretions (hydrocolpos). In some series, the incidence of a hydrocolpos approaches 40% (69,70). The hydrocolpos may compress the trigone of the bladder and interfere with drainage of the ureters. In addition, the vagina and uterus frequently suffer from different degrees of septation or even complete separation of two hemivaginas or two hemiuteruses. Patients with persistent cloaca have a high incidence of associated anomalies.
common channel (70). The length of the common channel varies between 1 and 10 cm. A short common channel is usually defined as shorter than 3 cm, whereas common channels longer than 3 cm are considered long-channel malformations. Figure 89-8A is the perineum in a female baby with imperforate anus, small genitalia, and single perineal orifice. Figure 89-8B shows a child with cloaca who has a common channel of approximately 3 cm. In contrast, Figure 89-8C shows a case in which the common channel is much longer. In this situation, separation and mobilization of the three structures, creating two walls out of each common wall between the urethra and the vagina, as well as between the vagina and the rectum, is difficult and most likely requires some form of vaginal replacement and probable laparotomy. Urinary function may be compromised in these long-channel defects. The perineum is usually well developed, and musculature, sacrum, and innervations are adequate in cases of short common channels. Frequently, the vagina in cases of persistent cloaca is distended and full of secretions (hydrocolpos). In some series, the incidence of a hydrocolpos approaches 40% (69,70). The hydrocolpos may compress the trigone of the bladder and interfere with drainage of the ureters. In addition, the vagina and uterus frequently suffer from different degrees of septation or even complete separation of two hemivaginas or two hemiuteruses. Patients with persistent cloaca have a high incidence of associated anomalies.
ANATOMY AND PHYSIOLOGY
For the pediatric surgeon, pertinent aspects of the anatomy and physiology of the anorectum should include knowledge of the normal process of defecation and continence and how these processes can be affected by the spectrum of ARMs. With respect to both defecation and continence, we must focus on the sphincter mechanism, anorectal sensation, proprioception, and finally, rectosigmoid motility. These areas comprise the functional elements of normal defecation and fecal continence. It is incumbent on the surgeon to realize how defecation and fecal continence can exist, given a spectrum of ARMs, a spectrum of anatomy, physiology.
Anatomy of Normal Children
Sphincter Mechanism
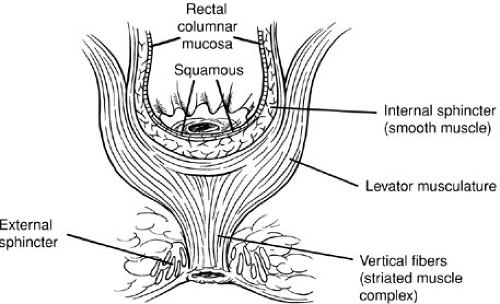
In normal children, the muscle groups of the sphincter mechanism include the voluntary striated muscles of the external sphincter and the levator musculature, and the involuntary, smooth muscle, internal sphincter (Fig. 89-9).
The striated external sphincter follows a circular path around the anus and is organized into three parts: subcutaneous, superficial, and deep (71) (Fig. 89-9). It is believed that the external sphincter is in a tonic state of contraction at rest. Because it can both relax and augment its baseline state of contraction at rest, it is possible that the striated muscle composition of the external sphincter is not homogeneous. This concept of differing cell types of striated muscle within the external sphincter, however, has not as yet been elucidated. The external sphincter is innervated by the pudendal nerve and the autonomic nervous system. The pudendal nerve is derived from the sacral plexus roots S2 to S4. This nerve is both motor to the external sphincter and sensory to the skin around the anus. Autonomic innervation of the external sphincter is via the nervi erigentes, derived from segments S2 to S4 of the spinal cord. This parasympathetic pelvic splanchnic nerve provides afferent information about the degree or magnitude of rectal fullness. Sympathetic innervation of the external sphincter probably exists, but no studies have yet identified its specific role.
The levator ani muscle is a series of striated muscle groups composed of ischiococcygeus, ileococcygeus, pubococcygeus, and puborectalis. The levator ani muscle extends from the pubic bone, the lowest portion of the sacrum, and the middle of the pelvis downward and medial to join with the external sphincter (71). This muscle group is usually depicted as a funnel-shaped sling. In contrast with the innervation of the external sphincter, the motor innervation of the levator musculature is from only S3 and S4. It is also innervated by the autonomic system (both sympathetic and parasympathetic).
The internal sphincter has been described as a continuation of the circular smooth muscle inner layer of the muscularis propia of the bowel wall (Fig. 89-9). The internal sphincter is under sympathetic (resting tone) and parasympathetic (relaxation) control (72).
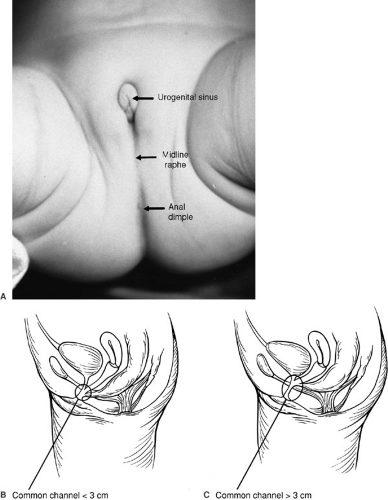 FIGURE 89-8. (A) Persistent cloaca, external topography. (B) Common channel cloaca shorter than 3 cm. (C) Common channel cloaca longer than 3 cm. |
Pain, touch, temperature, and pressure are felt through sensory afferents located in the anal mucosa, including a zone slightly more than 1 cm above the dentate line (73). The rectum is devoid of these sensory afferents except for pacinian corpuscles, which are pressure receptors located in the rectum between internal and external sphincters and in the presacral space and submucosa of the anal canal (74).
These anal mucosa sensory afferents traverse the spinal cord and then travel to the cortex, all with the feelings of fullness and ultimately a desire to defecate. Each sensory nerve ending is responsible for one kind of stimulus. Proprioceptive stretch receptors exist within the muscle spindles of the voluntary striated muscle mechanism. These receptors carry afferent impulses stimulated in response to rectal distention. These sensory afferents are derived from the neural crest in contrast with the mesoderm origin of the caudal eminence muscle, bone, and nerve (73,75).
Anatomy of Children with Anorectal Malformations
Children with ARMs challenge the traditional concept of the gross anatomy of the sphincter mechanism. After many years using the posterior sagittal approach to repair ARMs, it is our concept that the external sphincter runs in a paramedian direction rather than a circular direction. In addition, the muscular boundaries of the external sphincter are
fused; thus, we are unable to distinguish the components of the external sphincter.
fused; thus, we are unable to distinguish the components of the external sphincter.
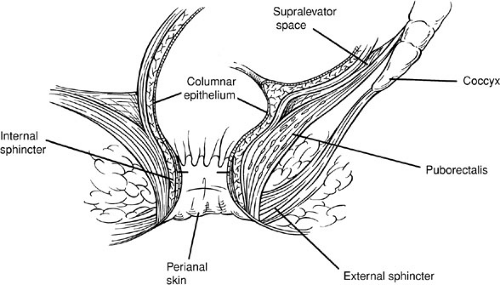
During a posterior sagittal dissection, the junction of levator musculature with external sphincter is defined by a vertical group of striated muscle fibers called the muscle complex (76) (Fig. 89-10). Electrical stimulation of the upper end of the levator group pulls the rectum forward. Stimulation of the muscle complex (vertical fibers) elevates the anus, and the paramedian fibers of the external sphincter close the anus.
Furthermore, in children with ARMs, there are varying degrees of striated muscle development from almost normal-looking striated muscle to virtually no muscle seen at operation. In very high defects, the rectum may rest at the upper part of the funnel-shaped voluntary striated muscle; in lower defects, the rectum may traverse the base of the muscular funnel.
Once again, the internal sphincter is said to be a continuation of the outer circular smooth muscle of the bowel wall. In children with ARMs, however, we have never seen what is referred to as the limits or, for that matter, the actual internal sphincter. Instead, if surgeons biopsy a segment of the wall of the rectum during an operative dissection for ARMs, they obtain histologic reference to smooth muscle. When subjected to electrical stimulation and drugs, this type of biopsy seems to respond as though it was, in fact, a bowel sphincter-type of smooth muscle; however, there has been no clear evidence for a definable internal sphincter in patients with ARMs (77).
Physiology of Normal Children
Continence in the normal child requires intact sphincter function, anal canal sensation and proprioception, and coordinated colonic and rectosigmoid motility. Unfortunately, much of the mechanism that underlies these events is unclear. Moreover, the movement of stool from the rectosigmoid down to the anus—namely, the process of
defecation—is even less well defined. As such, it is difficult to provide answers to pathologic conditions when normal physiology has not been elucidated.
defecation—is even less well defined. As such, it is difficult to provide answers to pathologic conditions when normal physiology has not been elucidated.
Sphincter Function
The two normally present types of sphincters include the voluntary external sphincter and the involuntary internal sphincter. Rectal manometric studies assume the internal sphincter provides the majority of resting pressure in the rectum by virtue of its near maximal contraction in the basal state (78). In response to rectal distention, during manometry, there is a fall in the pressure of the lower rectum and the anal canal that is called the relaxation reflex(79). It is assumed that relaxation of the internal sphincter provokes this fall in the intraluminal pressure. Relaxation is followed by a brief increase in pressure that is interpreted as a contraction of the external sphincter. This rectoanal inhibitory reflex (RAIR) is a manometric finding and may not be entirely extrapolated to defecation. In view of the fact that both structures (internal and external sphincters) are superimposed anatomically (Fig. 89-9), we do not understand how these conclusions were reached. In other words, how can we say that it is the internal or the external sphincter relaxing when they are both superimposed?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree