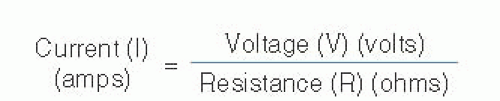Principles of Electrosurgery and Laser Energy Applied to Gynecologic Surgery
Ted L. Anderson
May S. Thomassee
DEFINITIONS
Active electrode—The electrode in monopolar circuits that carries the radiofrequency energy to the patient operative site.
Active electrode monitoring—A system with a sleeve placed around the active electrode to detect stray energy and to carry the induced energy to ground. Stray energy generally occurs from breaks in insulation or capacitive coupling.
Alternating current (AC)—Sinusoidal energy waveform (60 Hz) used in household electrical appliances and in electrosurgery.
Ammeter—Device that measures the amount of current flowing through a conductor at a specific moment.
Ampere—Quantity of electrons that move through a conductor over time (coulombs per second).
Bipolar—Closed circuit system where the active and passive electrodes are located within the energy device. This system does not use the patient as part of the circuit.
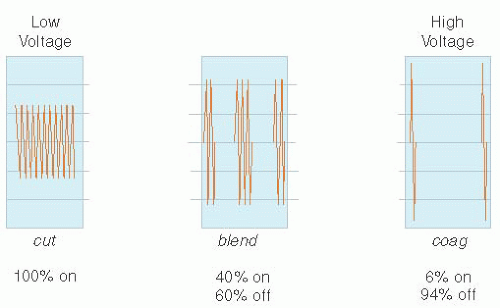
BLEND—Variation of electrical “on” and “off” time, where the current is interrupted at a variation of time, other than the standard CUT and COAG settings. (The current is “on” usually between 25% and 50% and varies with each BLEND setting.)
Capacitance—The buildup of electrical charge surrounding the active blade or even insulator of an electrosurgical device.
Capacitive coupling—Occurs when two conductors are separated by an insulator. Is always present, but not always dangerous. Becomes dangerous when the discharge of electrical energy occurs outside of the surgeon’s field of view or when it is not recognized as conducting energy to nearby tissue through electromagnetic current.
Circuit—An electrical network that has a closed loop giving a delivery and return path for electrical current, accomplishing work by routing electrons.
COAG—Function on electrosurgical unit to describe interrupted, modulated, or damped current. The voltage of this waveform is always, higher than it is with CUT waveforms, given the same power output.
Coulomb—Measure of a quantity of electrons.
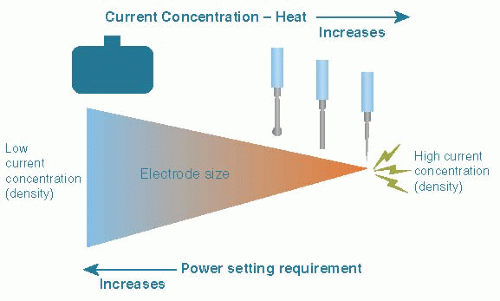
Current (power) density—Total amount of energy output per unit area of tissue. Affected by size of active electrode, shape, and power output (settings) of electrosurgical generator. Measured in watts (W). The smaller the spot of contact, the greater the density (current concentrated at the surface area of an electrode in contact with tissue during electrical flow) and the greater the heat effect for the same amount of time. Related to the square root of the area of contact.
Current (amperes)—Flow rate of a quantity (coulombs) of electrons.
CUT—Function on electrosurgical unit to describe uninterrupted, unmodulated, or undamped current in a continuous sinusoidal waveform. At the same power settings, the voltage of the waveform is always lower than it is with COAG or BLEND waveforms.
Desiccation—A form of coagulation achieved by “drying out” tissue through making contact with the active electrode. Either CUT or COAG waveforms may be used, but CUT waveform is preferable to reduce depth of penetration. Intracellular temperature stays below 100°C, which leads to cell shrinkage and dehydration.
Direct coupling—Occurs when two conductive materials in the same circuit touch during electrical activation or are close enough that arcing can occur. This can be intentional or unintentional. A break in the insulation of an active electrode that allows sparking to tissue is an example of unintended direct coupling.
Dwell time—Length of time an activated electrosurgical device is held at a specific tissue location.
Edge density—Affinity of electrons to concentrate at the edges of flat or irregularly shaped electrodes as they exit the electrode. This feature enhances the cutting ability of bladeshaped electrodes.
Electricity—Movement of electrons between two oppositely charged poles, positive and negative.
Electrocautery—Use of electricity to heat an object with subsequent direct transfer of energy by heat, such as a hot iron. Electrons do not move into the affected tissue; only heat is transferred.
Electrosurgery—Concentrated transfer kinetic energy (via electrons) from an active electrode to tissue creating a passive transfer of heat, using an electrosurgical generator.
Energy (joules)—Quantity of work produced over time. Energy (joules) equals work (watts) multiplied by time (seconds).
Faradic effect—Stimulation of tetanic muscle contractions, including cardiac muscle, when using electrical current with radiofrequency less than 100,000 cycles per second.
Fulguration—A form of coagulation achieved by arcing or spraying of “sparks” to tissue surface using high-voltage, damped, or interrupted (COAG) function with active electrode not touching tissue. Immediately causes charring and carbonization of the superficial tissue. Used to coagulate bleeding vessel or for treatment of endometriosis.
Heat (thermal energy)—Produced as electrons move from the low resistance of an electrosurgical probe to the high resistance of tissue. This energy may boil (vaporize) or denature (coagulate) tissue, depending on the extent and rapidity with which heat is generated.
Hertz (Hz)—Unit of measurement of electromagnetic sine wave. 1 Hz = 1 cycle per second.
Hybrid laparoscopic trocar sleeve—Conductive trocar sleeve used in laparoscopy that is covered by an outer nonconductive locking sleeve. Not used often anymore.
Impedance (ohms)—Resistance to flow of electrons through a conductor. Although resistance refers to direct current through a uniform wire, such as copper, it is generally substituted for impedance. Impedance is correctly applied with changes in voltage (alternating or fluctuating), frequency (modulating or demodulating), or tissue type (lipid membranes, soft tissue, fibrous tissue, fat, muscle, bone, or artificial appliances). It can measure the combination of tissue resistance and capacitance. Impedance in human tissue is generally 100 to 1,000 V; in the fallopian tube, it is 400 to 500 V.
Isolation ground circuitry—Safety feature that uses transformers not in contact with the parent generator so that the induced flow “floats” its own separate circuit. If a break in the floating circuit occurs, all energy within that circuit stops and does not seek ground.
Kilohertz (kHz)—Equal to 1,000 cycles of electromagnetic radio waves per second.
Kinetic energy—The energy that an object possesses due to its motion.
Monopolar—Type of electrode or electrical system in which the active electrode is small (high current density) and the passive electrode is large (low current density), and they are located remotely from each other. Most monopolar generators are calibrated against a 500-V load of resistance.
Open circuit (open “activation”)—State when the electrosurgical instrument is activated prior to touching the tissue. A charge density can build up at the tip of the electrode and spark or stray to an unintended site if activated for a prolonged period of time remote from the site of intended effect. Open circuitry is used to start fulguration.
Return electrode (passive or dispersive electrode)—Large conductive pad (low current density) placed on the patient to complete an electrosurgical pathway and return electrosurgical energy to the generator.
Return electrode monitoring—A system of modern electrosurgical generators whereby the return electrode consists of a dual pad system with internal monitoring capabilities to sound an alarm on the generator if not placed properly.
Radiofrequency—High-frequency electrical current in the range of 3 kilohertz (kHz) to 300 gigahertz (GHz), or 3,000 to 3 billion cycles per second.
Sparking (arcing)—Transmission of electrical energy through gas (air, argon). Used in a noncontact technique with COAG interrupted waveform for tissue fulguration.
Vaporization—Raising the cellular temperature rapidly above 100°C, which causes cell wall rupture, releasing steam. CUT mode is preferred for this with a noncontact technique.
Voltage (volts)—Electromotive force (pressure) that drives current.
Watts (work)—Amount of work produced by electron flow (current). Work (watts) equals force (volts) multiplied by current rate (amperes).
Waveform—The pattern of sinusoidal oscillation of an alternating electrical current from positive to negative.
Waveform frequency—Number of oscillations of an alternating electrical current, usually between 350,000 and 4 million cycles per second in electrosurgery.
INTRODUCTION
The practice of medicine and surgery has increasingly relied on applications of energy since the late 1800s. Indeed, the majority of gynecologic surgical procedures performed today incorporate some form of applied energy. However, the underlying physical principles that govern the desired biologic effects remain marginally understood by most surgeons. The typical resident graduating from an obstetrics and gynecology program has received limited formal training concerning the principles and application of electrosurgery, as was often the case for his or her faculty mentors. Importantly, these limitations in a surgeon’s knowledge of electrosurgical principles can permit delivery of unintended energy, resulting in immediate or delayed complications.
Over the past decade, electrosurgical instruments and generators have evolved into complex systems that can interact with biophysical properties of tissues to modulate, limit, and even discontinue energy delivery in response to measured parameters. In some cases, multiple energy modalities can be delivered by the same instrument. Thus, it is imperative that the contemporary gynecologic surgeon has a comfortable working knowledge of energy generation, delivery, and tissue effects in order to use these devices and systems effectively and safely.
Our goal in this chapter is to provide the basic fundamental principles of electrosurgery and laser technology. More specifically, we wish to provide a very practical approach that illustrates how these are applied within the field of gynecologic surgery to promote safe use of the available instruments.
HISTORY AND THE DEVELOPMENT OF ELECTROSURGERY
As early as the 4th century BC, the Egyptians described the treatment of wounds using a device called a “fire drill,” which turned rapidly to produce heat along its shaft. In the early writings of the Hippocratic Corpus (approximately 400 BC), followers of Hippocrates described the treatment of various tumors, as well as hemorrhoids, through direct application of heat. During this period, the use of heat was frequently accomplished through specific heating of a metal device and placing it directly on the wound, essentially inflicting third-degree burns without the ability to modulate tissue effect. Accordingly, the word “cautery” arose from the Greek term kauterion, meaning “hot iron.” Around 1600, the English physician and scientist William Gilbert introduced the term electricus meaning “like amber” as he discovered attraction of objects to each other after rubbing them against an amber rod. Once electricity was widely available, this concept was further expanded to “electrocautery.” Electrocautery is the use of electricity to heat the metal tip of a device and subsequently apply direct heat to the tissue. Thus, up until this point, all applications of heat to medicine were in the form of cautery or electrocautery.
It was actually Benjamin Franklin’s eighteenth century experiments with electricity that led to the idea that direct application of electrical current to tissue might be used to advantage in medicine. While John Wesley (England), Johann Kruger (Germany), and Jean-Antoine Nollet (France) experimented with paralytic conditions, Franklin and his Dutch colleague Jan Ingenhousz described a “highly elated state” after several unintended nonlethal shocks to the head and proposed this as therapy for melancholy.
Two significant discoveries paved the way for modern application of electricity in medicine. First was the recognition of electromagnetic induction by Michael Faraday and Robert Todd, leading to the ability to harness and store electrical energy reliably. This gave rise to a pathway for development of electrosurgical generators. The second was an extension of the work of Luigi Galvani, who demonstrated that electricity applied to frog legs induced muscle contraction, when William Morton and Arsenne D’Arsonval recognized application of electricity at a frequency of greater than 100 kHz allowed electricity to pass through the body without inducing pain or burn and without inducing muscle (including cardiac) spasm, the so-called faradic effect. D’Arsonval further noted that the current directly influenced body temperature, oxygen absorption, and carbon dioxide elimination, increasing each as the current passed through the body. Of note, the temperature was determined to increase proportionally to the square of the “current density.”
The French surgeon Joseph Rivière in the early 1900s was perhaps the first to use electricity clinically, in the form of an electrical shock to treat a hand ulcer. However, in the 1920s, it was Grant Ward who demonstrated that a continuous sinusoidal electrical waveform was superior for cutting tissue, and an interrupted electrical sinusoidal waveform resulted in more effective coagulation. This led to the now infamous collaboration between Harvey Cushing and physicist William Bovie to
produce an electrosurgical unit (ESU) (generator) designed to achieve intraoperative hemostasis during neurosurgical procedures. They published the results of a case series of intracranial tumor excisions in 1928, with an excerpt by Dr. Bovie describing the principles of superficial dehydration (desiccation), cutting, and coagulation as they applied to the tissue. These landmark events led to the era of modern applications of electricity in medicine.
produce an electrosurgical unit (ESU) (generator) designed to achieve intraoperative hemostasis during neurosurgical procedures. They published the results of a case series of intracranial tumor excisions in 1928, with an excerpt by Dr. Bovie describing the principles of superficial dehydration (desiccation), cutting, and coagulation as they applied to the tissue. These landmark events led to the era of modern applications of electricity in medicine.
BASIC PRINCIPLES OF ELECTROSURGERY
Electrocautery and electrosurgery are not synonymous. We distinguish between the two terms electrocautery and electrosurgery based on many differences as described in this chapter. Electrocautery refers to the application of electric current to an instrument of high resistance, resulting in heating, and then applying this hot instrument for direct transfer of heat to destroy tissue, without the ability to modify the depth of tissue penetration or tissue effect. For example, as described earlier, this would be like burning the skin with a hot iron. Conversely, electrosurgery is the employment of kinetic energy in the form of alternating current (AC) radiofrequency to transfer energy to tissue, raising intracellular temperature, which can be modulated to achieve desired tissue effects.
In order to achieve electrosurgery, there are three specific elements we must have. First, there must be a generator or ESU to accept electricity delivered from the electrical outlet on the wall of the operating room, modulate it to a higher frequency, and deliver it in the required conformation. Second, there must be an active electrode to deliver electricity to the tissue of interest in the form required. Third, there must be a return electrode to deliver the electricity away from the tissue to complete the electrical circuit.
The flow of electricity from an ESU through tissue follows the basic principles of physics. Particles of energy (electrons) are forced through tissue in a maximal direction from a positively charged pole to a negatively charged pole, in a sinusoidal waveform. The term circuit is used to describe the path the electrons take. In electric circuits, electricity is typically carried through conductors such as wire. However, electricity can also be carried through ion-containing substances like living tissue. Electron flow through cells creates changes in polarity of the cellular electrolytes (Na+, Ca++, K+, Cl–, etc.). Electromagnetic energy causes the anions to migrate toward the positive electrode and cations toward the negative, which is referred to as the galvanic effect. Importantly, the high-frequency flow of electrons in the radiofrequency spectrum surpasses that required for cellular membrane depolarization and does not affect the opening of sodium or calcium channels. Rather, the frictional forces of these charged intracellular ions create kinetic excitation and subsequent intracellular thermal heating as a result of thermodynamic changes.
The flow of electrons through a conductor is called current, which is governed by two opposing forces, namely voltage (the force pushing electrons along a circuit) and resistance (opposition to the free flow of electrons). This relationship is defined by Ohm law, which is depicted in Figure 15.1. You can see from this relationship that in order to increase electron flow (current), you must either increase the electromotive force (voltage) or decrease the impedance to free flow (resistance). It may help to think of this in terms of water flowing through a hose in your garden. If you kink the hose (increase resistance), your water flow (current) is going to decrease. The only way to accommodate for this is to increase the water pressure (voltage) proportionally.
We can further explore the relationship between resistance and voltage by examining the concept of power, defined as the instantaneous energy required per unit time to perform a function, measured in watts. Specifically, power is defined by the electromotive force (voltage) times the flow of electrons (current), or W = V × I. With mathematical substitution of Ohm law (I = V/R), we can derive that power (watts) is related to the voltage squared divided by resistance, or W = V2/R. In practical terms, this means that as resistance increases, in order to maintain the power required to perform a function, the electromotive force (voltage) must increase exponentially. As we shall see, it is the voltage that we must harness and control to accomplish electrosurgical tasks effectively and safely. If we go back to our hose analogy, this means that if you increase resistance (kink the hose), in order to maintain the watts or instantaneous energy required per unit time to perform a function of work (to water the garden), the voltage (water pressure) must increase. Therein, we have the basic mathematical and physical basis for applied electrosurgery.
ELECTROSURGICAL GENERATORS
Generators of ESUs deliver AC the surgical field carried by an electrosurgical instrument (active electrode). More specifically, the ESU must take the electrical current supplied from the wall outlet and change it to direct current. Then, through the use of oscillators, it must be modulated back to AC with higher frequency and the appropriate characteristics needed to produce the desired effects on tissues. The frequency delivered is between 500,000 and 5 million cycles per second and is sufficiently rapid to avoid stimulation of muscle contraction by surpassing the threshold for calcium and sodium channel depolarization. Because this frequency is in the range of AM radio waves, it is often referred to as radiofrequency (RF) current. Frequencies below 100,000 cycles per second are capable of causing tetanic muscle contraction, which is referred to as the faradic effect. On occasion, harmonic demodulation can occur, which produces small amounts of RF at less than 100,000 Hz presumably by alteration of current through interactions with the biophysical environment, which produces minor muscle twitches or nerve stimulation. Conversely, usual household appliances, such as hair dryers or blenders, use 60 cycles per second (or 60 Hertz, Hz) and are at much lower frequency than that of electrosurgical instrumentation (Fig. 15.2).
Most modern solid-state ESUs are capable of producing over 8,000 V, which is capable of pushing electrons up to 3 mm in room air under standard atmospheric conditions. However, more common outputs in typical use are in the 1,000 to 3,000 V range with a frequency upward of 350,000 Hz. Further, most generators today are calibrated to power output, with the power set reflecting the power available at the start of the electrosurgical application. As tissue impedance increases with heating in response to applied energy, we know from our prior calculations that power decreases. Additionally, many modern ESUs are best described as adaptive generators. Often designed to work in concert with specific instruments, they have the ability to adjust computer-controlled output in real time. They measure tissue impedance at the operative site and modulate output accordingly. Additionally, there are features for limiting maximum voltage, thereby reducing unintended effects of “stray energy.”
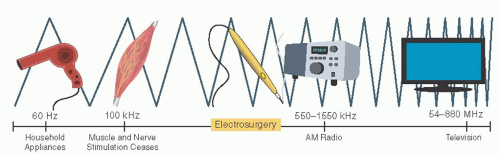 FIGURE 15.2 Radiofrequency spectrum. The frequency produced by electrosurgical generators overlaps with the range of AM radio waves and is thus referred to as “radiofrequency” (RF). |
Three fundamental principles that have guided the evolution of the modern ESU are “electricity must complete a circuit or it will not flow,” “electricity goes to ground,” and “electricity follows the path of least resistance.” Older generator models were ground referenced, which means that a “grounding pad” was required to return the electrical current delivered to the patient (complete the electrical circuit). However, given the other principles just mentioned, currents often traveled through alternative grounding pathways, including EKG clips, creating unintended patient thermal injury. Isolated ESUs were introduced in the late 1960s, whereby current delivered by the ESU was returned to the ESU, not to ground, to complete the circuit. Further, the current delivered to the patient was generated in transformers insulated from the ESU frame. Thus, when the electrical circuit is interrupted, the electrons do not seek ground; no current flows. This introduced the concept of “return electrode” rather than “grounding pad,” although the two terms are often (incorrectly) used interchangeably. This advancement dramatically reduced thermal injury hazards associated with earlier grounded systems. However, if return electrodes were not properly or completely placed, or if they began to peel off intraoperatively, burns could occur at these sites due to electrical arching and increased charge density.
Return electrode monitoring was introduced in the 1980s. In this system, still used today, return electrodes consist of two side-by-side conductive pads. Built-in monitors measure integrity of pad contact with skin and balance of contact between the two pads through a low-impedance feedback with the ESU. If there is an imbalance, poor contact, or a breach in contact, an alarm sounds and generator output is automatically discontinued. It should be noted that for these return electrodes to function effectively, both pads should be equal distance from the operative field with the largest edge facing the site.
MONOPOLAR AND BIPOLAR
All modern ESUs offer the ability to modulate electrical current output. The radiofrequency output can be delivered in monopolar or bipolar circuits (see Figs. 15.3 and 15.5 below). Further, radiofrequency can be delivered by providing a continuous or interrupted pattern RF energy. By convention, we typically refer to these two patterns as CUT and COAG (respectively) in homage to the description of tissue effects by Ward and Bovie in the 1920s.
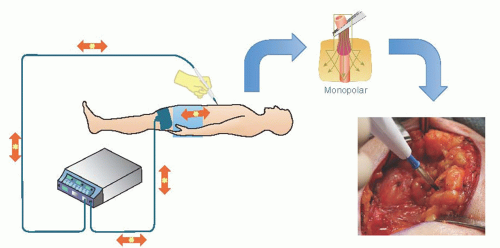
Of course, monopolar current is a misnomer, as all electrical circuits must be bipolar. The more appropriate distinction would be the location of the active and return electrodes with respect to each other. With monopolar circuits, the active electrode (instrument delivering RF energy) and the return electrode (sometimes called dispersive or passive electrode) are located remotely from each other. Thus, the RF energy enters the body (conductor) through the active electrode and is dispersed through a myriad of pathways following the path of least resistance to the return electrode to complete the electrical circuit (Fig. 15.3). The concentration
of RF energy at the active electrode is responsible for local tissue effect (e.g., burn) at that site. Conversely, the dispersed nature of RF energy through the body and at the site of the return electrode explains why there is minimal, if any, recognizable effect. This concept is known as “current density.” You may notice this concept readily when comparing the tissue effect using a standard electrosurgical spatula electrode with the edge versus the wide face of the blade facing the tissue. This is further illustrated by the increased tissue effect when using a needle tip electrode without decreasing the ESU output (Fig. 15.4).
of RF energy at the active electrode is responsible for local tissue effect (e.g., burn) at that site. Conversely, the dispersed nature of RF energy through the body and at the site of the return electrode explains why there is minimal, if any, recognizable effect. This concept is known as “current density.” You may notice this concept readily when comparing the tissue effect using a standard electrosurgical spatula electrode with the edge versus the wide face of the blade facing the tissue. This is further illustrated by the increased tissue effect when using a needle tip electrode without decreasing the ESU output (Fig. 15.4).
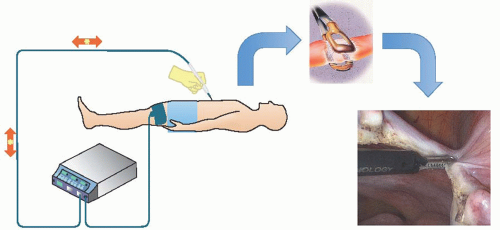
In bipolar circuits, the active and return electrodes are components of the same instrument. The charge density is basically identical at both electrodes. The only part of the patient involved in the circuit is that tissue directly located between the electrodes (Fig. 15.5).
In monopolar circuits, RF energy may be delivered either in a continuous waveform or in interrupted pulses electrical current, referred to as CUT or COAG, respectively, in deference to tissue effect described by Grant Ward in the 1920s. In CUT mode, there is delivery of a continuous uninterrupted sinusoidal waveform through the active electrode (continuous duty cycle). Alternatively, in COAG mode, the RF energy is delivered in pulses whereby over a given time RF energy is only delivered approximately 4% of the time (interrupted duty cycle). During the “off time,” desiccated, cooled, and coagulated tissue with denatured proteins increases resistance and thus increases voltage required for energy delivery. Most ESUs offer a “BLEND” mode in which the duty cycle is increased to 40%, but off 60% of the time, allowing for a mixture of cutting and coagulation properties (Fig. 15.6).
In bipolar circuits, RF energy is delivered by the ESU in CUT setting, which is a continuous sinusoidal waveform with low voltage. Modern instruments available to use in the bipolar mode also employ use of compressive force to reduce vascular pulse pressure and subsequently blood flow through the intervening tissue. This further helps the energy to remain concentrated between the electrodes in order to achieve maximal desired tissue effect. Further, there is often the incorporation of feedback mechanisms to determine when the intervening tissue is sufficiently desiccated. This feedback allows an adaptive ESU to recognize increased tissue resistance and discontinue energy delivery when complete tissue effect has been achieved.
TISSUE EFFECTS
Although the terms “CUT” and “COAG” have become ingrained in our electrosurgical lexicon, it is more useful to think of waveform and technique with respect to the tissue effect achieved. RF energy may be used to cut through tissue via rapid increase in temperature in a noncontact mode (vaporize) or coagulate tissue through slow deep dehydration and denaturation of proteins (desiccate) or by the superficial spray of electrons (fulgurate), often resulting in tissue carbonization (Table 15.1). Temperature changes have been identified with each of these effects. Normal resting human physiologic temperature is 37°C. Irreversible damage in tissue occurs at ≥50°C by intracellular protein denaturation and coagulation. Cellular dehydration (evaporation of water) occurs when tissue is heated to ≥90°C, which is referred to as desiccation. Rapid temperature rise to ≥100°C will cause cell walls to rupture as liquid water changes to steam by a process known as vaporization. At temperatures ≥250°C, tissues begin to char and carbonize leading to a fulguration effect.
As mentioned earlier, the CUT mode delivers a continuous sinusoidal waveform alternating from positive to negative at the frequency output of the ESU. This RF, delivered through a small active electrode (high current density), generates rapid and intense intracellular heat, which vaporizes the surrounding cells. The steam vapor occupies a space much greater than the water of the cell, creating two effects. First, it literally explodes the cells. Second, and equally importantly, it dissipates the heat generated to reduce thermal damage to adjacent tissue. Consequently, there is little or no coagulation effect. This mode is used to maximal advantage if the RF energy is engaged immediately before touching the tissue. If the active electrode is moved too slowly, or allowed to dwell in one spot too long, the tissue becomes dehydrated, resistance is increased, and tissue is more slowly dehydrated (desiccated). Therefore, for efficient and effective cutting of tissue, the surgeon should use a continuous waveform (CUT) with a small or thin active electrode that is activated just prior to tissue contact. With a peak voltage of about 200 V, the ionized air facilitates a layer of steam as the electrode glides by exploding cells with minimal surrounding heat or tissue coagulation.
TABLE 15.1 Tissue Effect Can Be Altered by Altering the Waveform and using the Active Electrode with a Contact or Noncontact Technique | |||
|---|---|---|---|
|