About 3 million venous thrombotic events, or venous thromboembolisms (VTEs), occur in the United States each year. About 2,000,000 plus of these events are deep vein thrombosis (DVT) in the hospital, and as many as 600,000 are pulmonary embolisms (PEs). Twenty-five percent of PEs are fatal, and VTE has been recognized as the biggest preventable cause of morbidity and mortality in United States hospitals. Ten percent of hospital deaths in the US are due to PE, and some patient groups, especially gynecologic malignancy patients, have a higher-than-usual risk for VTE, with a general incidence of about 15% to 20%. The risk of fatal postoperative PE among these patients is closer to 40% with no prophylaxis. More than 90% of PE patients have a lower or upper extremity DVT concomitantly.
PE has few defining characteristics, but the onset of respiratory distress compounded by hypotension, chest pain, and cardiac arrhythmias can be harbingers of impending death and are complications that convert an otherwise successful surgery into a postoperative fatality. Only 70% of patients who die of a PE have it considered in their differential diagnosis.
Modern diagnostic studies have provided more accurate information about the frequency of vascular complications and can identify those patients at risk of an embolic event. Pre- and postoperative prophylaxis with heparin or low molecular weight heparins (LMWHs) and concomitant use of embolic stockings and intermittent pneumatic compression (IPC) devices have significantly reduced the risk of VTE in the moderate- and high-risk patients. Clarke-Pearson and colleagues, using univariate and regression analysis, designed a prognostic model to evaluate the risk of postoperative VTE for an individual patient. In a group of 411 gynecology patients, the prognostic factors they identified included type of surgery, age, leg edema, non-Caucasian ethnicity, severity of varicose veins, previous radiotherapy, and a prior history of DVT.
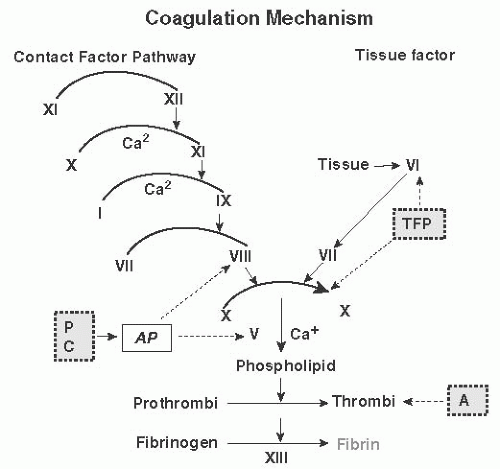
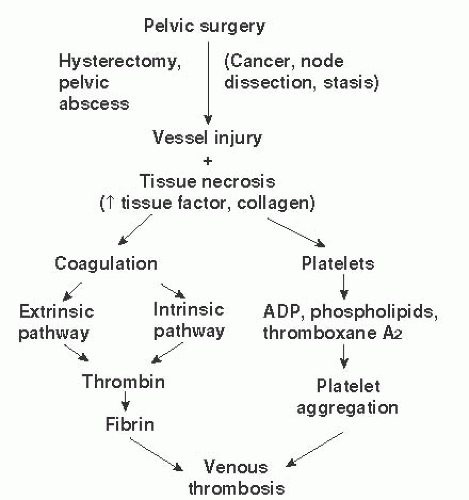
More than 130 years ago, Rudolph Virchow conceptualized the factors leading to postoperative thrombosis. These included venous stasis, changes in the blood constituents, and impaired function of the vessel wall. The blood clotting process is complicated (
Fig. 9.1), but it is initiated by the actions of tissue factor (TF) on factor VIIa after injury to vessels exposes the subendothelium and promotes platelet adhesion and aggregation to form a primary platelet plug. The process is completed by the actions of multiple components and factors in the blood that generate thrombin, the potent rate-regulating enzyme, which then interacts with fibrinogen and factor XIII to form an insoluble clot (
Fig. 9.2). Much recent evidence has focused on the role of cell-derived circulating microparticles (MPs) as potent etiologic agents for enhanced risk of VTE. This is because these MPs carry TF and other procoagulant phospholipids on their surfaces that potentiate activation of the coagulation pathways and may promote angiogenesis.
These tumorigenic effects on coagulation occur in addition to the typical acute postsurgical reactions seen in many hemostasis proteins. These include increases in fibrinogen, factor V, factor VIII, and von Willebrand factor, which promotes platelet adhesion and function. There is usually an increase in platelet number in the postoperative period, and the normal fibrinolytic response is blunted by the increase in PAI-1 and thrombin-activatable fibrinolysis inhibitor. Essentially, the fibrinolytic system is nonfunctional for several days following surgery, which down-regulates the ability of plasmin to impede wound healing by preventing degradation of fibrin and other matrix proteins.
Venous stasis is thought to be the cornerstone of postoperative thrombosis. Venous stasis in the pelvis and lower extremities results in platelet activation, promoting the adhesion of platelets to the endothelial cells lining the vessel, which are already stressed in a procoagulant mode. This results in conditions that encourage the development of a thrombus. These physiologic changes in venous hemodynamics occur in the pre-, peri-, and postoperative periods. Doran has shown that venous return from the lower extremities is decreased by half during surgical procedures because of the impact of muscle relaxation from anesthetic agents. Scanning using I125 fibrinogen has demonstrated that venous thrombosis is initiated during the surgery in 50% of patients who subsequently manifest a DVT. Lower extremity blood flow has been shown to decrease to about 75% of the normal drainage flow in the immediate postoperative period. This is an important reflection of Virchow triad on the role of adequate vessel flow. This reduction in flow persists for about 14 days after surgery because of the loss of muscle-pumping function in the legs. The major site of thrombus formation is the soleal venous sinuses of the calf, a portion of the venous arcade that joins the posterior tibial and peroneal veins draining the soleal muscle. Thrombi from these sinuses often occur posterior to valves located at the junction where these sinuses drain into the collecting veins. Thrombi often occur in these sinuses and in valve cusps in bedridden patients.
Diagnosis of Venous Thromboembolism
The traditional clinical methods used to diagnose venous thrombosis of the lower extremities are of limited value, with error rates approaching 50% for both false-negative and falsepositive rates. Most of the diagnostics problems occur because of the insidious nature of venous thrombosis in the lower extremity, which takes place in the soleal veins. Modern imaging methods have evolved considerably in recent years, ranging from I
125 fibrinogen scanning to venography to Doppler duplex ultrasound, impedance plethysmography (IPG), and magnetic resonance imaging (MRI) technique studies. Although for DVT venography remains the gold standard, modern compression ultrasonography is now the dominant technique, having a good negative predictive value (98%) for proximal DVT and slightly lower (
96%) for calf DVT. However, it is still inferior to venography, which remains the reference source (
Table 9.2).
Venography
The venogram has had the most extensive and rigorous use in clinical practice of all imaging techniques. However, it is no longer routinely used because it is invasive, uses contrast dye, has limitations, and provides an increased risk in many
patients who have renal compromise. However, the use of computed tomography (CT) venography in conjunction with CT angiography had proven to increase the sensitivity of DVT diagnosis from 83% to 90% in the PIOPED-II study.’
I125-Labeled Fibrinogen Scanning
This technique first developed in the 1960s was used widely for many years. It involves the intravenous injection of isotopelabeled fibrinogen, which is expected to be incorporated into the evolving thrombus and can be imaged by a scintillation scanner. Because of the use of isotopes, it is technically cumbersome and rarely used, despite many large studies validating its use in the 1970s and 1980s and its high correlation with venography.
Impedance Plethysmography
Impedance plethysmography is based on the principal of electrical resistance in specific areas of the body. When there is resistance to blood flow that is due to a thrombus, there is marked reduction in the electrical resistance over that vessel. IPG is most useful in proximal venous thrombosis but is relatively poor in visualizing thrombi below the knee because of the small caliber and slow flow rates through the soleal sinuses. The technique is only about 50% accurate compared with venography in detecting DVT below the popliteal vessels.
Huisman and colleagues evaluated 471 outpatients clinically suspected of acute-onset DVT. Four sequential IPGs were obtained on days 1, 2, 5, and 10 of the study. Of the 137 patients with abnormal results, 117 (85%) had abnormal results on day 1, with the other 20 patients becoming positive by day 10. When compared with venography, serial IPG had a specificity of 92% and a sensitivity of 100%. The use of serial testing clearly improved the ability to diagnose DVT. Another similar study by Vaccaro and associates involving 252 patients using single-test IPG gave a sensitivity of 84% and a specificity of 78%, confirming the superiority of the serial testing protocol. Given the short length of hospital stays now, it is unlikely that serial IPG would be possible despite its proven high correlation with venography.
Doppler Ultrasound
The use of Doppler ultrasound, often with computer color enhancement, has become the most widely used imaging technique for diagnosis of DVT. Its major physiologic use is in the measurement of flow velocity in larger blood vessels. In this technique, a reflected sound signal is converted to both an audible form and visual image on a computer screen. In the presence of a thrombosis, there is a decrease in the reflected signal that can be heard or, more likely, can be visualized. Most modern ultrasound machines use color enhancement to identify arteries (red) and veins (blue). This technique is again very useful to identify DVTs in the iliac, femoral, or popliteal veins, but its sensitivity falls off markedly when applied to the small vessels in the calf, usually to less than 60%.
Real-Time Ultrasound
Real-time ultrasound has been compared with venography, and in a study by Aitken and Godden, it demonstrated a sensitivity of 94% and a specificity of 100% in a small study of
46 patients. In a slightly larger study of 121 patients by Appelman and colleagues, the sensitivity was found to be
96%, and specificity was 97%.
Compression Ultrasound
This technique has been used in some large DVT trials, such as the PREVENT trial, but there have been few studies directly comparing it with venography. In a recent small study by Tomkowski and colleagues involving 160 medically ill patients, 12 patients had venographically proven DVT. Compression ultrasound technique had a sensitivity of 28% and a specificity of 98%, but despite the small numbers of venographically confirmed patients, the method performed poorly, having both false-positive and false-negative findings.
Duplex Doppler Ultrasound
This is a combination technique using real-time and Doppler methods in a procedure known as B mode or duplex Doppler imaging. It allows a radiologist to visualize the vessel and identify any thrombus in it. In a study by Langsfeld and colleagues, 431 patients were examined; 86 patients had a DVT. This gave a sensitivity of 100%, but two false positives dropped the specificity to 78%. Technical issues may have given one incorrect result, the second patient was pregnant, and the study was considered falsely positive because of aortocaval compression from the pregnant uterus.
In a study by Kristo and associates comparing duplex Doppler, venography, and single bilateral IPG, the respective sensitivities and specificities were as follows: ultrasound, 92% and 100%; venography, 100% and 75%; and IPG, 50% and 83%.
For many reasons, duplex B mode imaging has become the noninvasive imaging method of choice, essentially replacing venography as the “practical” gold standard.
Light Reflection Rheography
Light reflection rheography (LRR) uses infrared light directed at the skin. The backscattered rays are quantitated, which allow an estimation of blood volume. A decreased venous emptying rate of 0.35 is considered positive for DVT. In a study in patients with gastrointestinal problems in which 69 limbs were tested by venography and LRR, the sensitivity for LRR was 96%, the specificity was 83%, the positive predictive value was 79%, and the negative predictive value was 97%.
Light reflection rheography could prove to be a low-cost, sensitive tool for DVT detection. Further studies are needed to determine if the technique fulfills its early promise. However, in one study of 411 asymptomatic pregnant women in the second and third trimesters who did not have DVT, the use of LRR denoted a significant false-positive rate of 25% and an inadequate study rate of 19%. This gave an overall specificity of only 45%, indicating that LRR is not for use in DVT diagnosis in pregnant women.
Radioisotope Imaging
Various imaging methods have been tried using radioisotopes to try to detect thrombi in both arteries and veins by labeling components of the clotting system, such as platelets or fibrinogen. These labeled components became incorporated into the developing thrombus, and the focused radioactivity would then allow “visualization” by a detector. Radioactive-tagged antibodies to both platelets and factors have also been used for DVT diagnosis.
Indium111-labeled platelets have demonstrated good success with high sensitivity and specificity. The same is true for fibrinogen125– and technetium99m-labeled platelets.
Although all of these radioisotope-labeled methods have some proponents, the advances in computer software technology and imaging methods will probably outweigh significant development of radioisotopic methods for routine clinical VTE diagnosis.
Indirect Computed Tomography Venography
The new and evolving technique of indirect CT venography, using intravenous contrast medium injection followed by CT scanning of the limbs or chest, has a high potential for detecting DVT or PE. Some early studies have indicated detection of thrombi at least to calf level and perhaps lower. There are few studies comparing CT venography and CT pulmonary angiography. A recent study by Nchimi evaluated 1,408 patients with both techniques for PE detection and included lower-extremity DVT as a secondary finding. They found that in 48% of patients with DVT, the upper end of the thrombus was between the ankle and the knee. Comparing the two techniques, CT venography detected 17% more VTE than did CT pulmonary angiography.
Magnetic Resonance Imaging/Magnetic Resonance Imaging Venography
The use of MRI techniques with or without contrast media is another new approach to VTE detection. There are several different technologies using MRI, but all basically use the differences in signal intensities to distinguish flowing blood from stagnant blood (i.e., clot). Although there have been few largescale studies published, the major advantage of MRI is that no contrast medium is required, allowing the technique to be used in pregnant women. One study by Carpenter and colleagues indicated no statistical differences between contrast venography and MRI in DVT diagnosis. Magnetic resonance imaging techniques for routine VTE and arterial thromboses diagnosis will likely continue, as improvements in computer software will provide for the advancement of MRI-based method as tools for vascular diagnostics.
Evolving Imaging Techniques
Because of progress in instruments and computer software in conjunction with ongoing concerns about radiation exposure to patients, several new technical modifications are being evaluated, especially for PE diagnosis. These include multidetector computed tomography angiography (MDCTA), electrocardiogram (ECG)-gated CT angiography, and dual-energy/dual-source CT angiography. Early studies suggest that these methods, especially the dual-source CT angiography, may have significantly enhanced accuracy using the very latest detection technology to minimize the radiation required to provide good diagnostic images.
Nonimaging Methods
The use of laboratory tests as a means of exclusion of VTE has gained momentum in the last decade. The use of the automated quantitative D-dimer assays has gained widespread acceptance, especially in emergency rooms, to exclude VTE. In a study by Wells and associates comparing IPG and D-dimer with contrast venography, the combination of IPG and a negative (normal) D-dimer test gave a negative predictive value of 97%. In this same study, the combination of positive IPG and positive D-dimer had a positive predictive value of 93% for any DVT and 90% for proximal DVT.
The use of an appropriate D-dimer assay in isolation has been shown to have about 98% negative predictive value for exclusion of DVT. Despite numerous studies, there is still no proven consistent correlation between a positive D-dimer assay and the presence of venous thrombosis.
The utility and power of D-dimer assays for exclusion of PE and DVT have been enhanced by the use of pretest probability scores. Several of these exist, namely, the Wells model criteria for DVT and PE diagnosis as well as the Geneva score or other modifications such as the Pisa score. All of these scoring systems are simple and applicable to most emergent VTE diagnostic situations. In conjunction with D-dimer assays, they all enhance the negative predictive value for VTE exclusion and hence the need for unnecessary imaging studies.
Because the diagnosis of PE can be difficult in many older patients, especially those with heart failure or other cardiovascular complications, some preliminary studies have been done using combinations of D-dimer, B-type natriuretic peptide, and cardiac troponins to determine if a better distinction can be made between a PE and an underlying cardiac complication. These studies are likely to be the forerunners for other combinations of biomarker lab tests to identify more specific negative or even positive predictive markers for VTE. This is especially important since many older patients with VTEs have some degree of renal impairment, and the use of contrast dyes in imaging can be problematic.
Risk Factors for Vascular Complications
Several clinical factors are known to identify the patient with an increased risk for VTE (
Table 9.3). The most prevalent and important include age >40 years, obesity >20% above ideal weight, prolonged surgery, and immobility in the pre-, peri-, and postoperative periods. Pelvic malignancy, prior VTE, known thrombophilia risk, severe diabetes, heart failure, prior radiation therapy, and chronic obstructive pulmonary disease all increase the VTE risk.
Age
An autopsy study by Sevitt and Gallagher demonstrated that DVT was most prevalent in patients older than 60 years. Several studies have shown a linear risk of fatal PE with increasing age. Approximately 10% of hospitalized patients’ deaths are due to PE, and only about 35% of these are diagnosed ante mortem. Contributing factors include degenerative changes in the vascular tree, increases that occur in the concentration of many coagulation factors, and, possibly, increased platelet adhesiveness.
Immobility
Prolonged inactivity in the preoperative patient promotes an impairment of venous flow in the lower extremities. Many diagnostic techniques also produce a decrease in muscle tone with a secondary decrease in venous flow. These hemodynamic changes promote sludging of red cells and activation of platelets, setting the stage for VTE during the operative period. This is one of the main risk factors described by Virchow for the etiology of thrombosis.
Studies done using I125 fibrinogen scanning presurgery and immediately postsurgery have indicated that in 50% of patients who subsequently developed VTE, the initiation of clot formation occurred during the surgical procedure. This is amplified during prolonged anesthesia, with generalized muscle relaxation further promoting venous stasis in the lower extremities, which compounds the thromboembolic risk. For this risk, the judicious use of prophylactic anticoagulation in the high-risk patient should include the operative phase and continue at a minimum until the patient is fully ambulatory.
Postoperative immobility also promotes VTE risk by continuing venous stasis, and studies have shown that 66% of patients who develop a DVT do so in the first 48 hours after surgery. Other compounding issues include sitting with legs crossed or dangling over the bed or the exaggerated Fowler position. These positions all produce impairment of lower extremity venous return. Postoperative patients should be ambulated early and aggressively; if ambulation is not possible, they should have their legs elevated to 15 degrees above the horizontal.
Other Factors
Other factors include previous VTE, varicose veins, severe diabetes, cardiac failure, chronic obstructive pulmonary disease, and underlying thrombophilia. Given the high incidence of factor V Leiden (5%) and the G20210A prothrombin gene mutations (3%) in the Caucasian populations, which are wellrecognized risk factors for venous thrombosis (
Table 9.4), these genetic risk factors are common enough to make a major contribution to preoperative thrombosis, even in the patient with no prior VTE history.
Underlying malignancy is a huge contributor to risk, most likely because of the significant up-regulation of TF that is known to occur with many malignancies. Up-regulation of TF increases thrombin generation by several mechanisms, promotes platelet activation, and enhances the generation of MPs and angiogenesis, as discussed earlier.
A review of these risk factors clearly identifies the high-risk VTE patient, and it is essential that these surgical risk variables are identified and understood in designing appropriate thromboprophylaxis and monitoring parameters to prevent venous thrombosis. Many countries including the United States have produced national consensus documents from their clinical
oncology societies for both treatment and prophylaxis for VTE in cancer patients.
Prophylaxis
Prevention remains the most effective tool in the treatment of VTE. Between 5% and 45% of gynecologic surgery patients develop DVT in their legs; of these, 20% have popliteal or femoral involvement; and of these, 40% will progress to PE with its high mortality. It is imperative that methods for prophylaxis are planned and implemented before surgery (
Table 9.5).
In the Sixth American College of Chest Physicians (ACCP) Consensus Conference, Geerts and colleagues—in a review of PE in 7,000 gynecologic surgery patients in prospective clinical trials—reported a reduction in the rate of fatal PE of 75% using thromboprophylaxis (
Table 9.6). The current 2012 Chest (ACCP) guidelines recommend the routine use of LMWH or unfractionated heparin (UFH) and mechanical compression stockings given the high risk in this population. They do not recommend the use of inferior vena cava (IVC) filters.
Low-Dose Unfractionated Heparin
Low-dose UFH has been the mainstay of prophylactic treatment for many years, with numerous prospective randomized clinical trials validating a risk reduction in DVT incidence from 35% to 45% to about 7% in the high-risk patient. In one large study by Kakkar and colleagues (
Table 9.7) incorporating 4,000 patients at multiple centers, patients were randomized to 5,000 U USP calcium heparin subcutaneously starting 2 hours before surgery and subsequently every 8 hours thereafter for the next 7 days. The reduction in VTE between the control group (25%) and the treatment group (8%) was highly significant. The most important finding was the decrease in fatal PE from 16 patients in the control group to 2 in the treatment group confirmed by autopsy.
Because there is no change in the activated partial thromboplastin time (APTT) because of the low level of subcutaneous heparin, there was no increase in postoperative bleeding. This is because the main impact of the low-dose heparin is exerted via antithrombin through factor Xa, as well as directly on thrombin. There is also a secondary effect in which heparin releases tissue factor pathway inhibitor (TFPI), which also helps to down-regulate factor Xa by forming a complex involving TF:F Xa:F VIIa:TFPI.
These studies, as well as those shown in
Table 9.8, clearly validate the efficacy of low-dose UFH in reducing VTE in surgical patients using an initial dose of 5,000 U 2 hours before surgery and then 5,000 U every 12 hours for the next 5 days. For the truly high-risk patient, such as those with a prior VTE or multiple risk factors, 5,000 U every 8 hours should be used. In current clinical practice, the use of UFH has been superseded by the use of LMWHs.
Low-Dose Unfractionated Heparin/Dihydroergotamine
The combination of low-dose UFH and dihydroergotamine (DHE) treatment was shown to work well by adding the known effect of DHE as a selective venous vasoconstricting agent to the anticoagulant properties of UFH.
Dextran 70/Dextran 40
In 1972, Bonnar and Walsh described the use of dextran 70 to prevent thrombosis after pelvic surgery. A subsequent study by Bernstein and colleagues involving radical hysterectomy patients using dextran 70 as prophylaxis showed a decrease in DVT incidence from 33% to 5%. Dextran works by interfering with platelet function, interacts with factor V and VIII, and inhibits fibrinolysis. Despite some comparable studies between dextran and low-dose UFH, the Sixth ACCP Consensus Conference in 2001 recommended against using dextran products in VTE prophylaxis.
Low Molecular Weight Heparins
Low molecular weight heparins (LMWHs) act by primarily inhibiting factor Xa with a small component of activity against thrombin. They have become mainstay of anticoagulant prophylaxis and treatment and continue to replace all other forms of drug therapy. The drugs have a longer half-life than does UFH and are much more biopredictable. If LMWH levels are measured in patients using an anti-Xa assay, there is a remarkable homogeneity of response. This has led the U.S. Food and Drug Administration to recommend against the need to monitor when LMWHs are used for VTE prophylaxis. There are currently four LMWH drugs available in the United States (Fragmin, Lovenox, Innohep, and Arixtra). They are subtly different in molecular weights and in manufacturing processes but in essence are almost identical in clinical efficacy. They are not, however, dosed in the same way, some using milligrams and other units, or even units per kilogram. Pharmacists can provide accurate dosage information about any of the products available.
Multiple studies reported in the literature essentially show equivalence or better for the LMWHs compared with UFH and/or Coumadin in the prevention of VTE, but almost all of these show a much lower bleeding risk for the LMWH treatment groups, even with hard data for bleeding risk being quantitated by transfusion requirements. LMWHs have also been compared with dextran and used in combination with DHE with good outcomes, but the reality is that in normal clinical practice, LMWHs by themselves provide adequate protections with minimal complications. One other advantage of the LMWH preparations is their much lower incidence of heparin-induced thrombocytopenia (HIT) when used as de novo therapy. However, if a patient has had HIT in the past, these preparations should not be used because there is a 90% cross-reactivity between UFH and LMWH for the antibody causing HIT. The one exception to this is Arixtra, the synthetic factor Xa inhibitor, which has been shown to cause clinical HIT in two to three cases to date.
It is highly likely, given the once-per-day dosage requirement and the lack of HIT risk, that LMWHs will continue to dominate for thromboprophylaxis therapy in VTE.
Newer Oral Anticoagulants
In the past 18 months, three new oral anticoagulants have been approved for use in various medical conditions. One of these drugs, dabigatran (Pradaxa), is an anti-IIa (thrombin) inhibitor approved for atrial fibrillation similar to a new oral anti-Xa inhibitor apixaban (Eliquis). However, the third new drug, an oral anti-Xa inhibitor, rivaroxaban (Xarelto), has been approved for treatment and prophylaxis of VTE although not specifically tried in cancer-related VTE.
The fact that Xarelto is oral, does not require any monitoring, and has few side effects is likely to promote increased use especially from the patient compliance perspective where the need for subcutaneous injections remains a problem.
Compression Modalities
As long ago as 1944, Stanton et al. used static compression to decrease venous stasis by decreasing the luminal diameter of the veins, thereby increasing blood flow velocity. In the mid-1970s, Sigel and colleagues showed an increase in blood velocity of 20% using graduated compression stockings but a 200% increase in velocity using intermittent sequential compression.
Mittelman and colleagues showed that uniform intermittent calf compression was not as effective as intermittent sequential compression at increasing thigh blood flow. This is another example of a component of Virchow triangle, namely, stasis being involved in the VTE protection mechanism.
It is possible that another component of the triad—the coagulation system—is also influenced by IPC because several groups have shown that it stimulates fibrinolysis, perhaps by increasing prostacyclin production. Prostacyclin is a potent natural vasodilator and antiplatelet agent released from endothelial cells. Guyton and colleagues found increased quantities of 6-keto prostaglandin F
1α in patients undergoing IPC compared with controls. The 6-keto prostaglandin F
1α is a
specific breakdown product of prostacyclin. Frango and associates have shown a 16-fold increase in prostacyclin production in cultured endothelial cells submitted to pulsatile shear stress compared with a twofold increase with contact shear stress.
Graduated Compression Stockings
Initial studies evaluating antiembolic stockings proved inconclusive and relied on several different methods to diagnose VTE, which compounded the uncertainty. Sigel and colleagues designed a compression thromboembolism deterrent (TED) hose with graduated pressures of 18, 14, 12, 10, and 8 mm Hg from the ankle to the upper thigh. Scurr and associates evaluated TED hose in a study of 70 patients older than 40 years undergoing major abdominal surgery in which only one leg had TED hose applied. Using I125 fibrinogen scanning as the diagnostic tool, 19 patients had DVTs in the control leg, and only 1 had a DVT in the TED hose leg. A subsequent similar study by Inada and colleagues found a DVT frequency of 14.5% in the control leg and only 3.6% in the TED leg. Malignancy is a powerful predisposition to VTE secondary to stasis and tissue factor production by the tumor. In a study by Allan and associates assessing the efficacy of TED stocking in patients undergoing abdominal surgery for malignant and benign diseases, the incidence of DVT in the benign disease group was 24.5% in the control limb and 6.1% in the TED limb. However, in the malignant disease group, the incidence of DVT was only slightly increased in the control limb at 27.9%, but the incidence of DVT in the TED limb was significantly higher at 11.5%, clearly amplifying the impact of the tumor on the DVT risk. The Sixth ACCP Consensus Conference suggested that TED hose with early ambulation was an acceptable and effective means of VTE prophylaxis in the low-risk gynecology surgery patient.
External Intermittent Pneumatic Compression
These techniques also promote increased blood flow in the lower extremities that is due to decreased stasis and improved fibrinolysis. Nicolaides and colleagues compared intermittent sequential pneumatic compression, nonsequential (one chamber) pneumatic compression, and UFH in the prevention of DVT. Using pressures of 35, 30, and 20 mm Hg sequentially for 12 seconds at the ankle, calf, and thigh, respectively, they observed a 240% increase in peak blood velocity. In contrast, using the singlechamber device at 35 mm Hg, the increase was only 180%. The intermittent sequential device was more effective than was the single-chamber device and was as effective as 5,000 U of UFH every 12 hours in preventing DVT. In addition, the intermittent sequential device increased the time interval for clot formation proximal to the calf compared with UFH. In another study, the same authors compared electrical calf stimulus, low-dose UFH, intermittent sequential compression, and TED hose in 150 patients older than 30 years undergoing major abdominal surgery. The incidence of proven DVT was 18%, 9%, and 4%, respectively.
In a similar study in patients undergoing surgery for gynecologic malignancy comparing no thromboprophylaxis to nonsequential external compression, the control group had a VTE frequency of 34.6%. In the compression treatment group, the VTE incidence was reduced to 12.7%. Diagnostic tools for VTE were IPG and I131.