Pericardium and Great Vessels
Walter Pegoli Jr.
Pediatric Surgery, University of Rochester School of Medicine and Dentistry, Rochester, New York 14642.
PERICARDIUM
Embryology
The pericardial sac begins to take shape early in the fourth week of gestation. Clefts in the embryonic mesoderm develop and ultimately separate the somatic and splanchnic components. These individual clefts coalescence and form a single cavity. The central region of this cavity becomes the pericardial space, and the lateral aspects eventually form the pleural cavities. The floor of the pericardial cavity contains a layer of splanchnopleure that develops into the cardiogenic plate. This plate ultimately forms the myocardium and the visceral pericardium.
Early in gestation, the pericardial cavity is large, and the pleural cavities are relatively small. With development of the fetal lungs, the size of the pleural cavities increases rapidly. Abnormalities in the formation of the pleuropericardial membrane result in pericardial defects. These defects are attributable to abnormal organogenesis during the fifth week of gestation. Most abnormalities are located on the left side.
Anatomy
The pericardium surrounds the heart and is lined by simple squamous epithelium or mesothelium. It has both visceral and parietal layers. The visceral pericardium is intimately associated with the heart; the parietal pericardium forms the sac. The two components of the pericardium are contiguous at the sites where great vessels enter and exit the heart. The area between the visceral and parietal pericardia is the pericardial space. The pericardial space is filled with a small volume of serous fluid that acts as a lubricant.
The anterior pericardium separates the heart from the sternum. The posterior pericardium is adjacent to the esophagus and thoracic aorta. Superiorly, the pericardium separates the heart from the thymus. The inferior pericardium is contiguous with the central tendon and anterior aspect of the diaphragm. Laterally, the pericardium and the parietal pleura are in intimate contact.
The blood supply to the pericardium is derived from branches of the internal thoracic arteries and the descending thoracic aorta. The venous drainage is through the azygous system. The sympathetic trunks and the phrenic and vagus nerves innervate the pericardium. Lymph drains through the thoracic duct and the right lymphatic duct. The absorptive capacity of the pericardium is limited. Therefore, situations that increase fluid formation, such as inflammatory states, can lead to pericardial effusions.
Physiology
The pericardium performs several important functions. It fixes the heart anatomically within the thorax, it minimizes the friction between the heart and surrounding structures during the cardiac cycle, it prevents mechanical distortion of the great vessels, and it helps prevent spread of infection from contiguous sites. Because collagen is the major structural component, the pericardium has a limited ability to distend acutely. When the contents of the pericardial sac exceed a particular volume, a point of pericardial nonextensibility is reached, and the pericardium limits cardiac filling and therefore cardiac output. This is the physiologic basis for tamponade. Clinical evidence for tamponade includes the Beck triad: (1) elevated central venous pressure (distended neck veins); (2) a small, quiet heart (diminished QRS-complex amplitude); and (3) systemic hypotension. A paradoxical pulse may be demonstrated on physical examination. Experiments in dogs have shown that hydrostatic distending pressures of 3 to 8 mm Hg can produce a noncompliant pericardium and result in tamponade (1). In children, the most frequent condition that produces a pericardial effusion that exceeds pericardial reserve volume is a viral infection. Chronic pericardial effusions of considerably greater size can be tolerated without physiologic decompensation because the pericardium is
capable of modest distention without hydrostatic pressure increases under these conditions.
capable of modest distention without hydrostatic pressure increases under these conditions.
Anatomic Defects
Anatomic defects in the pericardium are uncommon and usually occur on the left side. These defects communicate with the ipsilateral pleural space. If the defect is large, segments of the heart or the entire heart can be located within the pleural space. More often, a small portion of the heart is herniated into the pleural space, most commonly the auricular appendage. The phrenic nerve usually courses along the anterior margin of these smaller defects.
Pericardial defects are often associated with cardiac anomalies; the most common are the tetralogy of Fallot, patent ductus arteriosus (PDA), mitral valve prolapse, and mitral stenosis. Bronchogenic cysts and enteric cysts are also associated with these defects. The Cantrell pentalogy is present when pericardial defects are associated with diaphragmatic defects, abdominal wall defects, lower sternal defects, and intracardiac abnormalities.
Most patients with pericardial defects do not have symptoms. Chest pain, shortness of breath, dizziness, and hemoptysis can occur with cardiac displacement, however, and sudden death with cardiac herniation and strangulation has been described.
On physical examination, patients exhibit a systolic murmur, accompanied by a shift of the point of maximal cardiac impulse to the side of the defect. Electrocardiographic findings can include right-axis deviation, incomplete right bundle branch block, or right ventricular hypertrophy. On plain chest radiograph, there is elongation of the left heart border. Echocardiography is routinely done, but is relatively nonspecific. The finding of a left atrial aneurysm should raise the possibility of a partial pericardial defect. The diagnosis of a pericardial defect is most reliably established with computed tomography (CT) or magnetic resonance (MR) imaging.
Patients with large or complete pericardial defects have little or no potential for herniation and strangulation, and are not considered for surgical intervention. Patients with partial defects are at risk for cardiac herniation and sudden death. These patients should undergo surgical reconstruction by enlarging the defect, resecting the pericardium, or patching the defect with prosthetic material or local tissue transfer.
Pericardial Cysts and Diverticula
Diverticula or cystic sequestrations of the pericardium typically occur at the cardiophrenic angle. Like the pericardium itself, they are lined by simple squamous epithelium and filled with serous fluid.
Pericardial cysts and diverticula have a male predominance and are twice as likely to occur on the right side. Ninety percent are unilocular; the remainder are multilocular. Although most patients with these findings do not have symptoms, precordial or substernal chest pain, dyspnea, and chronic nonproductive cough have been noted. Complications of pericardial cysts are rare. As with any benign cystic lesion, infection, rupture, and compression of adjacent structures from local enlargement can occur. Malignancy is rare. These cysts are not known to regress spontaneously.
On plain chest radiograph, the typical pericardial cyst appears as a smooth, rounded mass in the region of the cardiophrenic angle. The differential diagnosis includes foramen of Morgagni hernia, mediastinal cystic hygroma, and mediastinal teratoma. As for most thoracic structural anomalies, contrast-enhanced CT is a sensitive diagnostic tool. The lesion is seen as a thin-walled oval or tubular structure filled with fluid. It typically displaces rather than infiltrates surrounding structures.
Surgical excision is advised for these lesions. Resection is both diagnostic and prophylactic. When symptomatic, cysts should certainly be removed. The blood supply is limited so the cyst can be excised without difficulty. Diverticula require ligation and removal. This is technically straightforward and either a conventional transthoracic or a thoracoscopic approach can be used (2). Aspiration has been performed in high-risk patients, using either fluoroscopic or ultrasonographic guidance; however, recurrence is an important concern with this approach. In one 3-year follow-up of a small group of patients who underwent aspiration, no recurrences were reported (3).
Infections
The classic clinical triad for acute pericarditis includes chest pain, pericardial friction rub, and electrocardiographic abnormalities. The most common causes of acute pericarditis in children are viral infection, bacterial infection, uremia, and trauma. Precordial pain is characteristic of the acute condition. The onset usually coincides with fever, but can follow a shaking chill. The pain is usually intensified by respiration, coughing, swallowing, or lying the supine position. In some patients, the pain is diminished by the assumption of an upright position.
Specific Forms of Viral Pericarditis
Acute viral pericarditis can follow infection with the coxsackievirus B, echovirus 8, mumps, Ebstein-Barr virus, influenza, poliomyelitis, or varicella virus. Patients with infectious mononucleosis can present with acute pericarditis and cardiac tamponade followed by progressive pericardial restriction. Viral pericarditis evokes significant inflammation. The inflammatory process can be serous, serofibrinous, fibrinous, suppurative, hemorrhagic, or some combination of these. Viral pericarditis is usually of the fibrinous or serofibrinous variety; suppurative pericarditis is often the result of bacterial infection. With resolution of the acute process, the fibrin either undergoes fibrinolysis or becomes organized to obliterate the
pericardial space. The latter outcome is more frequent after severe infections.
pericardial space. The latter outcome is more frequent after severe infections.
Patients with viral pericarditis usually have an antecedent history of an upper respiratory tract infection. Seventy percent of patients have temperatures as high as 39°C. Pleuritic chest pain, a pericardial friction rub, cough, and a pericardial effusion are common. The pericardial fluid typically is clear and serous, resolving spontaneously in most cases.
A specific virus rarely can be retrieved from pericardial fluid, stool, or blood. The diagnosis is commonly established by demonstrating a fourfold increase in serial neutralizing antibody titers in serum. The electrocardiogram may demonstrate sequential ST-T-segment and T-wave changes. Sinus tachycardia is often present (4). Atrial and ventricular arrhythmias result from inflammation of the underlying myocardium. In otherwise healthy children, the differential diagnosis should include blunt chest trauma, systemic lupus erythematosus, rheumatic pericarditis, and bacterial endocarditis.
Patients with viral pericarditis have no symptoms for about 1 to 2 weeks. Those with coxsackievirus or echovirus infections are at particular risk for myocarditis with cardiac insufficiency and cardiomegaly. The cardiac dysfunction can resolve completely or result in persistent physiologic dysfunction that can take the form of chronic ventricular failure or even sudden death. Patients with coxsackievirus infections are at risk for the development of coronary artery aneurysms and therefore require vigorous and regular follow-up.
The basic treatment for patients with acute viral pericarditis is bed rest and analgesics. Specific antiviral agents are not available for the responsible pathogens. During the acute phase, patients should be observed for evidence of cardiac tamponade, myocarditis, and heart failure. Patients with significant pain are treated with a 3- to 7-day course of systemic steroids. In patients with mild symptoms, aspirin or indomethacin are adequate. This therapy generally results in the rapid resolution of symptoms within 12 to 24 hours.
Bacterial Pericarditis
Children who present with bacterial pericarditis commonly have a history of acute pharyngitis, pneumonia, meningitis, otitis media, anemia, impetigo, or purulent arthritis. The most common organism is Staphylococcus aureus, followed in frequency by Haemophilus influenzae type B, Neisseria meningitidis, other gram-negative organisms, Streptoccocus pneumoniae, and β-hemolytic streptococcal organisms.
Bacterial pericarditis presents as an acute illness with high fever, shaking chills, dyspnea, night sweats, and cough. Chest pain is an uncommon symptom. Patients can manifest tachycardia, a pericardial friction rub, pulsus paradoxicus, and in severe cases, systemic hypotension. The white blood cell count usually exceeds 17,000 cells per μL, with a shift toward immature forms on the differential count.
A plain chest radiograph may show findings that correlate with the cause. In particular, pulmonary parenchymal disease, pneumonia, pleural effusion, or mediastinal widening may be evident radiographically. Nonspecific ST-T-wave changes are present on electrocardiogram. The leukocyte count of the pericardial fluid is usually greater than 50,000 cells per μL, consisting mostly of polymorphonuclear leukocytes; the glucose level is usually less than 35 mg per dL; and the protein content is more than 3 g per dL (5).
Antibiotic therapy alone is inadequate treatment for acute bacterial pericarditis. Mortality rates are reduced by surgical drainage combined with appropriate antibiotic treatment. The initial antibiotic coverage should include an agent effective against S. aureus and an aminoglycoside. Patients with a penicillin allergy should receive systemic vancomycin. Either closed or open drainage should be instituted as outlined later, and early pericardiectomy should be considered to prevent the development of constrictive pericarditis.
Surgical Intervention
Pericardiocentesis
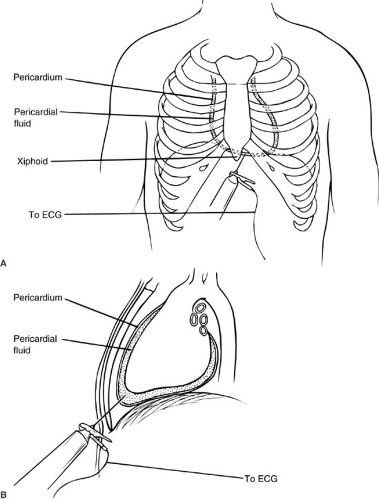
Aspiration of pericardial fluid can be a life-saving maneuver in patients with cardiac tamponade, regardless of the cause. In addition, it is an indispensable adjunct to the diagnosis and treatment of idiopathic pericardial effusions. The most common approach is a subxiphoid route. The procedure is optimally done with full cardiac monitoring. A long needle is attached to a syringe and electrocardiograph lead. The needle is usually inserted to the left of the xiphoid and directed to the ipsilateral shoulder posteriorly at a 45-degree angle. The needle is slowly advanced until fluid is retrieved or until electrocardiographic changes occur (Fig. 63-1). An adjunct to this procedure is to use this needle as a guide for placing a catheter into the pericardial space. This can be done by threading a catheter through the needle, or by placing a wire through the needle and using the wire to direct a larger catheter. This latter technique is especially valuable in patients who had recurrent effusions requiring repeated pericardiocentesis.
The potential risks of pericardiocentesis include pneumothorax, myocardial or coronary artery injury, and arrhythmias. The use of sonographic guidance techniques has lessened the incidence of these potentially life-threatening complications.
Pericardiostomy
Open drainage of the pericardium is most commonly required in cases of malignant effusions or acute pyogenic pericarditis. The former is rare in children. For the latter,
this procedure may be necessary if the effusion is fibrinous or fibrinopurulent and has multiple loculations. Classically, this procedure was done through an open subxiphoid approach that allowed dependent drainage while avoiding contamination of the pleural space.
this procedure may be necessary if the effusion is fibrinous or fibrinopurulent and has multiple loculations. Classically, this procedure was done through an open subxiphoid approach that allowed dependent drainage while avoiding contamination of the pleural space.
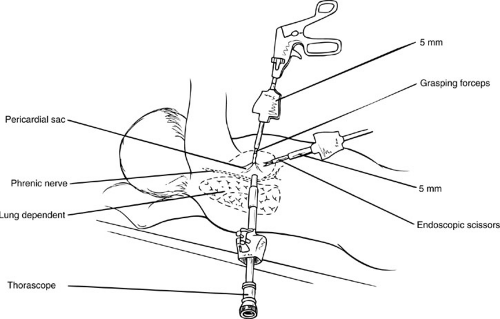
Pericardiostomy can be performed using thoracoscopic techniques (6). The patient is positioned supine at 45 degrees to allow the lung to fall posteriorly after pneumothorax has been achieved. Access ports are placed in the seventh intercostal space, midaxillary line; fourth intercostal space, midclavicular line; and sixth intercostal space, anterior axillary line (Fig. 63-2). The thoracoscope is inserted through the port in the seventh intercostal space. Dissection using monopolar current is avoided because it can fibrillate the heart and result in cardiac arrest if used in proximity to the myocardium. The laser or bipolar cautery is recommended for hemostasis. Segments of the lateral pericardium can be excised under direct vision. During dissection, it is important to identify the vagus and phrenic nerves on the pericardial surface. An advantage of the thoracoscopic approach is complete visualization of the pericardium. Three-dimensional video imaging technology and the use of the harmonic scalpel improve efficiency and precision (7). The ability to identify both the phrenic and vagus nerves, improved cosmesis, and less postoperative discomfort are additional benefits. The major disadvantage is that drainage is into the pleural cavity. When this is an undesirable clinical outcome, such as with bacterial pericarditis, an alternative route is indicated.
Pericardiectomy
Open resection of the pericardium is performed for patients with constrictive pericarditis. The procedure can be
performed through either a median sternotomy or an anterolateral thoracotomy. Cardiopulmonary bypass may be an advantage in that the heart can be manipulated to a greater degree. In addition, the posterior, lateral, and diaphragmatic aspects of the pericardium can be resected, which is difficult via conventional thoracotomies. The major risks in the use of cardiopulmonary bypass are systemic heparinization and concomitant bleeding. The principal alternative approach is the anterolateral thoracotomy, usually through the left fifth intercostal space. The advantage is that cardiopulmonary bypass is not required, and the risk of bleeding is therefore diminished.
performed through either a median sternotomy or an anterolateral thoracotomy. Cardiopulmonary bypass may be an advantage in that the heart can be manipulated to a greater degree. In addition, the posterior, lateral, and diaphragmatic aspects of the pericardium can be resected, which is difficult via conventional thoracotomies. The major risks in the use of cardiopulmonary bypass are systemic heparinization and concomitant bleeding. The principal alternative approach is the anterolateral thoracotomy, usually through the left fifth intercostal space. The advantage is that cardiopulmonary bypass is not required, and the risk of bleeding is therefore diminished.
GREAT VESSELS
Embryology
The earliest embryonic blood vessels develop from yolk sac mesoderm. These take the form of arborizing cords of angioblasts. The heart forms ventral to the foregut. Paired aortic arches pass laterally from the ventral heart, around the gut, and eventually unite to form the dorsal aorta. During the fifth week of development, a series of paired vessels that supply the six branchial clefts form, segments of which develop into the aortic arch and its major branches. Nearly all important anomalies of the aorta and its branches can be explained by the abnormal growth and involution of one or more portions of the primitive arch system. The six pairs of arches are not all present simultaneously. The first and second arches develop initially, and most segments undergo involution. The remnants of the first branchial arch form part of the mandibular artery. Remnants from the second arch form the hyoid artery and arteries of the inner ear. A large portion of the third arch remains and eventually forms part of the common carotid artery and the internal carotid artery. The right fourth arch forms the proximal portion of the right subclavian artery, and the distal segment is derived from a portion of the right dorsal aorta. A remnant of the left fourth arch forms the segment of the aorta between the origin of the left common carotid artery and the left subclavian artery. The fifth aortic arch pairs are transient and regress early. The right sixth arch forms the proximal right pulmonary artery; the proximal left sixth arch develops into the proximal left pulmonary artery, and the distal portion remains as the ductus arteriosus.
In the embryo, the heart and great vessels begin as cervical structures that later migrate caudally. On rare occasions, the aortic arch fails in this migration and remains in the cervical position, forming a cervical aortic arch. This structure is an anatomic curiosity and typically produces
no symptoms, but this possibility should be considered in the evaluation of a pulsatile neck mass.
no symptoms, but this possibility should be considered in the evaluation of a pulsatile neck mass.
Critical events in the embryologic development of the great vessels occur during the eighth week of gestation. A double aortic arch results if the left and right fourth arches both persist. If the left fourth arch regresses and the right fourth arch persists, a right aortic arch results. Abnormal segmental regression of the left fourth arch results in abnormalities in the aorta that range from coarctation to complete interruption of the aortic arch.
Anatomy
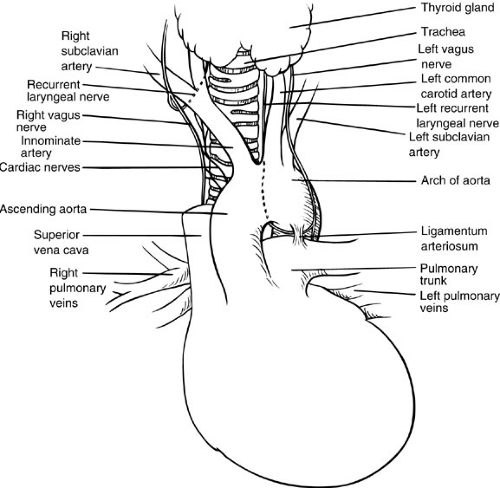
The ascending aorta originates at the heart and travels through the pericardium, where it emerges to become the aortic arch. The superior vena cava lies to the right, and the pulmonary artery crosses posterior to the ascending arch. The aortic arch passes anterior to the trachea, giving rise to the brachiocephalic trunk or innominate artery, the first of three great arteries originating from the aortic arch. The brachiocephalic artery divides promptly into the right common carotid artery and the right subclavian artery. The second great branch of the aortic arch is the left common carotid artery, which runs almost vertically cephalad between the left pleural sac and the trachea to the base of the neck. The third and most distal artery arising from the aortic arch is the left subclavian artery, which passes along the cephalad aspect of the left pleural sac. As it gives rise to its three great branches, the anterior aspect of the arch of the aorta is covered by the left brachiocephalic vein. The aorta normally passes to the left of the trachea. The left phrenic nerve and the vagus nerve cross the anterior aspect of the aorta. On the right, at the level where the vagus nerve lies directly anterior to the subclavian artery, the nerve gives rise to the right recurrent laryngeal nerve, which passes posterior and then cephalad, thereby encircling the right subclavian artery. At about the level of the origin of the left subclavian artery, the aortic arch is joined on its inferior border by the ligamentum arteriosum extending from the left pulmonary artery. The vagus nerve on the left side courses anterior to the arch of the aorta in the vicinity of the ligamentum arteriosum and gives off the left recurrent laryngeal nerve, which passes around the arch in intimate contact with the junction of the aorta and ligamentum arteriosum (Fig. 63-3).
Anomalies of the Aortic Arch
During the past several decades, surgical correction of the major anomalies of the aortic arch has become routine.
Although some malformations are asymptomatic, patients with potentially life-threatening obstruction of the trachea or esophagus can be offered a predictably curative surgical procedure in most instances (8,9).
Although some malformations are asymptomatic, patients with potentially life-threatening obstruction of the trachea or esophagus can be offered a predictably curative surgical procedure in most instances (8,9).
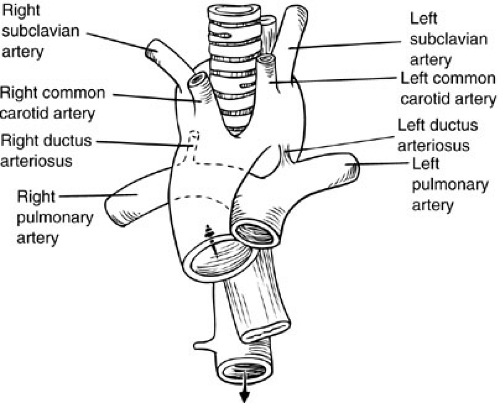 FIGURE 63-4. Schematic illustration of double aortic arch encircling esophagus and trachea.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|