Pediatric Spleen Surgery
Henry E. Rice
Division of Pediatric Surgery, Duke University Medical Center, Durham, North Carolina 27710.
ANATOMY
The spleen arises as a condensation within the dorsal mesogastrium at 5 to 6 weeks’ gestation. With continued growth, the spleen is carried into the left upper quadrant of the abdomen. The convex smooth surface of the spleen faces superiorly, posteriorly, and to the left in relation to the abdominal surface of the diaphragm. The costodiaphragmatic recess of the pleura extends down as far as the inferior border of the normal-size spleen.
At birth, the spleen normally weighs between 10 and 12 g, and its growth parallels body weight. The normal spleen size and weight vary somewhat between children, and closely correlate with the size of the child (1). One rule of thumb is for assessing normal splenic size is that the spleen and left kidney are the same length as measured by ultrasonography. Using two standard deviations above the mean as a guide, the upper limit of normal for the spleen/kidney ratio is 1.25 (1).
The visceral relationships of the spleen are with the greater curvature of the stomach, the tail of the pancreas, the left kidney, and the splenic flexure of the colon. The parietal peritoneum is firmly adhered to the splenic capsule, except at the splenic hilum. The peritoneum extends superiorly, laterally, and inferiorly to form the suspensory ligaments of the spleen. The splenorenal ligament extends from the anterior left kidney to the hilum of the spleen and contains the splenic vessels. These layers continue superiorly to the greater curvature of the stomach to form the two leaves of the gastrosplenic ligament through which the short gastric arteries and veins course.
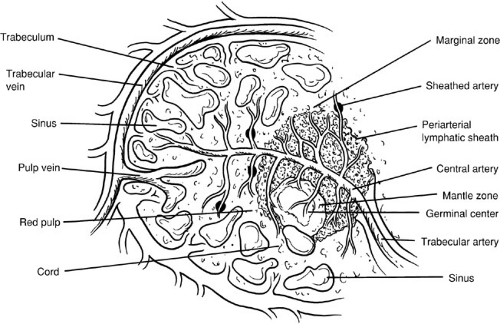
The splenic artery arises from the celiac trunk and courses along the superior border of the pancreas. The branches of the splenic artery include the pancreatic branches, short gastric arteries, left gastroepiploic artery, and terminal splenic branches. The splenic artery divides into several branches within the splenorenal ligament before entering the splenic hilum, where they branch again into these trabeculae as they enter the splenic pulp (Fig. 94-1). Two variations of the splenic vasculature exist, as described by Michels in 1942(2). In the distributed pattern, multiple branches arise from the main trunks approximately 2 to 3 cm from the hilum. In the magistral pattern, the pedicle formed by the artery and vein enters the hilum as a compact bundle.
As small arteriolar branches branch into the spleen, their adventitial coat becomes replaced by a sheath of lymphatic tissue. It is these lymphatic sheaths that comprise the white pulp of the spleen and that are interspersed along the arteriolar vessels as lymphatic follicles. The interface between the white pulp and the red pulp is known as the marginal zone. As the arterioles lose their sheaths of lymphatic tissue, they traverse the marginal zone and enter the red pulp, which is composed of large branching, thin-walled blood vessels called splenic sinuses and sinusoids.
The venous sinusoids empty into the veins of the red pulp, and these veins drain back along the trabecular veins that empty into major tributaries, ultimately joining to form the splenic vein. The splenic vein runs inferior to the artery and posterior to the pancreatic tail and body. The splenic vein joins the superior mesenteric vein behind the neck of the pancreas to form the portal vein. The inferior mesenteric vein often empties into the splenic vein; it may also empty into the superior mesenteric vein at or near the confluence of the splenic vein and superior mesenteric vein.
VARIANTS OF NORMAL ANATOMY
Congenital asplenia (Ivemark syndrome) arises from a failure of normal organogenesis and is a consequence of
bilateral right-sidedness. It can be associated with bilateral trilobed lungs and a centrally located isomeric liver. It is often associated with heterotaxy syndromes, and the stomach is often located in the right side of the abdomen. Asplenia has a high rate of association with complex congenital heart disease, with the most common combinations including total anomalous pulmonary venous return, common atrium, atrioventricular canal, double outlet right ventricle, and pulmonary stenosis or atresia (3). Functional asplenia can be diagnosed by the presence of Howell-Jolly bodies on a peripheral blood smear.
bilateral right-sidedness. It can be associated with bilateral trilobed lungs and a centrally located isomeric liver. It is often associated with heterotaxy syndromes, and the stomach is often located in the right side of the abdomen. Asplenia has a high rate of association with complex congenital heart disease, with the most common combinations including total anomalous pulmonary venous return, common atrium, atrioventricular canal, double outlet right ventricle, and pulmonary stenosis or atresia (3). Functional asplenia can be diagnosed by the presence of Howell-Jolly bodies on a peripheral blood smear.
Congenital polysplenia occurs with multiple spleens located along the greater curvature of the stomach. Similar to asplenia, it is often associated with situs inversus and cardiac defects. Importantly, polysplenia can be associated with other surgical conditions, such as biliary atresia and preduodenal portal vein. Despite the numerous splenic remnants, splenic immune function is generally normal.
A wandering spleen (splenic ectopia) refers to the spleen being mobile and present anywhere in the abdomen other than the left upper quadrant. Although its etiology is not well defined, it is probably the result of failure of the fusion of the dorsal mesogastrium to form the suspensory ligaments of the spleen. It generally presents with the sudden onset of abdominal pain related to torsion, although chronic or intermittent pain can also occur. Therapy is individualized based on the presence or absence of perfusion to the spleen at the time of presentation and exploration—if the spleen has torsed and is nonviable, splenectomy is indicated. If functional splenic tissue remains, splenopexy in the normal anatomic position is required.
SPLENIC FUNCTION
The spleen has important hematopoietic functions during early fetal development, and along with the liver, is a major site of red and white blood cell production. By the fifth month of gestation, the bone marrow assumes the predominant role in hematopoiesis, and normally there is no significant hematopoietic function left in the spleen. The spleen continues to function throughout gestation and after birth for blood filtration, removal of intracellular material, and immunologic defense.
The filtration functions of the spleen are closely linked to its unique vascular anatomy. The arteries flow through the white pulp (lymphoid tissues), after which most of the blood flow enters the macrophage-lined reticular meshwork and the blood flows back to the venous circulation through the venous sinuses. Blood elements must pass through slits in the lining of the venous sinuses; if they cannot pass, they are trapped in the spleen and ingested by splenic phagocytes. Experimental animal studies have demonstrated that an intact splenic arterial system is necessary for optimal control of infection (4).
The mechanical filtration of the spleen is essential for the processing of immature and senescent erythrocytes.
Normal red blood cells (RBCs) are biconcave and deform relatively easily to facilitate passage through the microvasculature. As immature RBCs pass through the spleen, they undergo several types of repair, including removal of nuclei and excessive cell membranes, converting them from a spherical nucleated to a biconcave mature morphology. In cases of anatomic or functional asplenia, there are characteristic alterations in the morphologic appearance of the peripheral RBCs. These include the presence of target cells (immature cells), Howell-Jolly bodies (nuclear remnant), Heinz bodies (denatured hemoglobin), Pappenheimer bodies (iron granules), stippling, and spur cells. Aged RBCs that have lost membrane plasticity are trapped and destroyed in the spleen.
Normal red blood cells (RBCs) are biconcave and deform relatively easily to facilitate passage through the microvasculature. As immature RBCs pass through the spleen, they undergo several types of repair, including removal of nuclei and excessive cell membranes, converting them from a spherical nucleated to a biconcave mature morphology. In cases of anatomic or functional asplenia, there are characteristic alterations in the morphologic appearance of the peripheral RBCs. These include the presence of target cells (immature cells), Howell-Jolly bodies (nuclear remnant), Heinz bodies (denatured hemoglobin), Pappenheimer bodies (iron granules), stippling, and spur cells. Aged RBCs that have lost membrane plasticity are trapped and destroyed in the spleen.
In children with various congenital hemolytic anemias, abnormal erythrocytes are trapped by the splenic filtering mechanism, resulting in worsening anemia, symptomatic splenomegaly, and splenic infarction. In autoimmune hemolytic anemias, IgG bound to the cell membrane targets the RBCs for splenic destruction by splenic macrophages. A similar IgG-dependent mechanism is involved in platelet destruction in immune thrombocytopenic purpura (ITP). The mechanical filtration of the spleen is important for the clearance of circulating pathogens that reside within erythrocytes, such as malarial parasites or bacteria such as Bartonella species. Mechanical filtration by the spleen is also essential for the removal of unopsonized, noningested bacteria from the circulation, and may be particularly important for clearing microorganisms for which the host has no specific antibody.
Finally, the spleen is a major site of production for the opsonins properdin and tuftsin, and removal of the spleen results in decreased serum levels of these factors. Properdin can initiate the alternative pathway of complement activation to produce destruction of bacteria, as well as foreign and abnormal cells. Tuftsin is a tetrapeptide that enhances the phagocytic activity of both polymorphonuclear leukocytes and mononuclear phagocytes. The spleen is the major site of cleavage of tuftsin from the heavy chain of IgG, and circulating levels of tuftsin are suppressed in asplenic subjects (5). Moreover, neutrophil function is decreased in asplenic patients, and the defect appears to result from the absence of a circulating mediator (6).
These immune functions of the spleen contribute to the maintenance of normal host defenses against certain types of infectious agents. It is well established that people lacking a spleen are at a significantly higher risk for overwhelming postsplenectomy infection (OPSI) with fulminant bacteremia, pneumonia, or meningitis, as compared with those with normal splenic function (7). Major pathogens in OPSI include organisms such as Streptococcus pneumoniae, in which polysaccharide capsules requiring both antibody and complement are important in host defense against these organisms. Asplenic subjects have defective activation of complement by the alternative pathway, leaving them more susceptible to infection.
OVERWHELMING POSTSPLENECTOMY SEPSIS
OPSI is among the more devastating sequelae associated with functional or anatomic asplenia, and is the most common fatal late complication of splenectomy (7,8,9,10,11). The association between removal of the spleen and sepsis has been recognized since 1919, although attention was refocused on this condition by King and Shumaker in 1952 (9). They reported sepsis in five infants who had undergone splenectomy, and subsequent reports have confirmed the association of overwhelming sepsis with functional or anatomic asplenia.
The exact incidence of OPSI has been difficult to determine, and the incidence of infection in postsplenectomy patients is likely underreported. However, the risk of fatal OPSI appears to be greater in young children (younger than 4 years of age), and may be as high as up to 1 per 300 to 350 patient-years follow-up (7,8,9,10,11). Although the risk of overwhelming postsplenectomy sepsis is reduced by use of immunizations to S. pneumoniae, Meningococcus, and Haemophilus influenzae, as well as postoperative antibiotic prophylaxis, its risk is never eliminated (10). Moreover, concerns persist of incomplete protection by pneumococcal vaccinations, antibiotic resistance, and poor compliance with antibiotic prophylaxis (10,11).
OPSI typically begins with a prodromal phase characterized by fever and chills and nonspecific symptoms, including sore throat, malaise, myalgias, diarrhea, and vomiting. Patients may have had symptoms for 1 to 2 days before seeking appropriate medical treatment. Pneumonia and meningitis may be present, but many cases have no identifiable focal site of infection and present with high-grade primary bacteremia. Progression of the illness can be quite rapid, with the development of hypotension, disseminated intravascular coagulation, respiratory distress, coma, and death within hours of presentation. The mortality rate is between 50% and 70% for fully developed OPSI, and this high mortality persists despite current use of broad-spectrum antibiotics and intensive care. Survivors of severe OPSI often have a long and complicated recovery, with severe sequelae such as extremity gangrene, deafness from meningitis, mastoid osteomyelitis, bacterial endocarditis, and cardiac valvular destruction.
The spleen is important for generating responses to thymus-independent antigens. Prior to a planned elective splenectomy, immunizations against Pneumococcus, Meningococcus, and H. influenzae should be administered whenever possible (Fig. 94-2). The American Academy of
Pediatrics has recommended that the immunization precede splenectomy by at least 2 weeks (12). In cases of trauma requiring splenectomy in which presplenectomy immunizations are not possible, immunizations should be administered to patients prior to discharge from the hospitalization in which their splenectomy occurred, rather than waiting until a follow-up visit. Many of these patients become lost to follow-up, and clinical studies have demonstrated adequate antibody response to immediate immunization (13). Simultaneous immunization with H. influenzae type b, Meningococcus, and polyvalent pneumococcal vaccine is both immunogenic and well tolerated.
Pediatrics has recommended that the immunization precede splenectomy by at least 2 weeks (12). In cases of trauma requiring splenectomy in which presplenectomy immunizations are not possible, immunizations should be administered to patients prior to discharge from the hospitalization in which their splenectomy occurred, rather than waiting until a follow-up visit. Many of these patients become lost to follow-up, and clinical studies have demonstrated adequate antibody response to immediate immunization (13). Simultaneous immunization with H. influenzae type b, Meningococcus, and polyvalent pneumococcal vaccine is both immunogenic and well tolerated.
ANTIBIOTIC PROPHYLAXIS
Postoperative antibiotic prophylaxis is generally recommended for all young children after splenectomy, and some authorities have advocated this form of prophylaxis in older children and adults, although data showing the efficacy of this treatment are lacking (14). Several clinical trials have supported the use of antibiotic prophylaxis in specific populations of young children who are increased risk for pneumococcal infection, such as those with sickle cell disease. For example, antibiotic prophylaxis efficacy against invasive pneumococcal infections has been demonstrated in children with sickle cell disease in a prospective multicenter, randomized, double-blind trial of penicillin administration (125 mg of penicillin VK, administered orally twice daily to 3 years of age, and 250 mg twice daily thereafter) (15). A 84% decrease was observed in the incidence of pneumococcal infection in the antibiotic prophylaxis group.
Problems with the use of orally administered penicillin in children have included reports of breakthrough invasive infections and low compliance (16). In addition, several investigators have demonstrated an increase in penicillin-resistant strains of pneumococci in children with sickle cell disease (17). The specific effect of antibiotic prophylaxis on reduction of infection risk, compliance with prophylaxis, and effects on nasopharyngeal colonization with pneumococci have not been studied specifically in children after surgical splenectomy (14).
The exact length of time to continue antibiotic prophylaxis for children after splenectomy is unclear. A multicenter study of children with sickle cell disease examined the safety of discontinuing penicillin prophylaxis after 5 years of age (18). Children who had received at least 2 years of penicillin prophylaxis before their fifth birthday and one dose of pneumococcal polysaccharide vaccine were randomized to receive continued prophylaxis or placebo. There was no difference in the rate of invasive pneumococcal infection among the children receiving penicillin prophylaxis and those receiving placebo—4 cases (2%) and 2 cases (1%), respectively. The small numbers of enrolled children and the low incidence of pneumococcal disease limit the interpretation of these data. In general, most young children continue antibiotic prophylaxis for several years after splenectomy, although the ideal duration for continued prophylaxis is unclear.
Available data do not support the practice of long-term penicillin prophylaxis in asplenic adults. Another
approach that appears rational is to provide the asplenic patient with a supply of oral antibiotics, with instructions to begin taking the medication at the onset of rigor or a febrile illness if appropriate medical evaluation is not immediately available. Fever and rigor in an asplenic patient should prompt immediate aggressive empirical treatment with antibiotic coverage, even in the absence of culture data.
approach that appears rational is to provide the asplenic patient with a supply of oral antibiotics, with instructions to begin taking the medication at the onset of rigor or a febrile illness if appropriate medical evaluation is not immediately available. Fever and rigor in an asplenic patient should prompt immediate aggressive empirical treatment with antibiotic coverage, even in the absence of culture data.
DISORDERS OF THE SPLEEN
Hereditary Spherocytosis and Other Erythrocyte Membrane Disorders
Hereditary spherocytosis (HS) is caused by a defect in the RBC membrane that affects the spectrin component (19). The characteristic finding is increased numbers of spherocytes in the peripheral blood. HS is the most common cause of hemolytic anemia in people of Northern European heritage, with a prevalence of 1 in 5,000. The defect in the RBC membrane is not uniform and may affect one or more membrane components. In the classic autosomal dominant form, the defect may be in beta spectrin, ankyrin, or protein 3. In the recessive form, the defect is in either the alpha spectrin or protein 4.2.
The cardinal features of HS are anemia, jaundice, and splenomegaly. Some patients may be asymptomatic and are detected only on family screening. Anemia is the most common presentation (50% of cases); each of the other clinical features may be present at diagnosis in 10% to 15% of cases. During the course of the illness, about 50% of patients develop jaundice, and 50% develop a palpable spleen in the first year of life. Anemia is much more likely to be severe in early childhood than later in life. The reticulocyte count is elevated generally, and the peripheral smear may show spherocytes in up to 80% of cases and occasionally nucleated RBCs. The definitive diagnostic test is the incubated osmotic fragility test, which shows a pattern of increased fragility in HS.
Splenectomy decreases the rate of hemolysis and usually leads to resolution of the anemia (20). Classic indications for splenectomy in HS are severe anemic crises and repeated transfusion requirements. It is generally recommended that the operation be delayed until after the fourth or fifth year of life to preserve immunologic function of the spleen in young children who are most at risk for OPSI (20).
Other anemias associated with erythrocyte structural abnormalities include hereditary elliptocytosis, hereditary pyropoikilocytosis, hereditary xerocytosis, and hereditary hydrocytosis. These conditions result in abnormalities of the erythrocyte cellular membrane and increased RBC destruction. Splenectomy is indicated for severe anemia that commonly occurs in these conditions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree