Pancreas
Sheilendra S. Mehta
George K. Gittes
Department of Pediatric Surgery, Children’s Mercy Hospital, Kansas City, Missouri 64108.
Department of Pediatric Surgery, Children’s Mercy Hospital, Kansas City, Missouri 64108.
EMBRYOLOGY
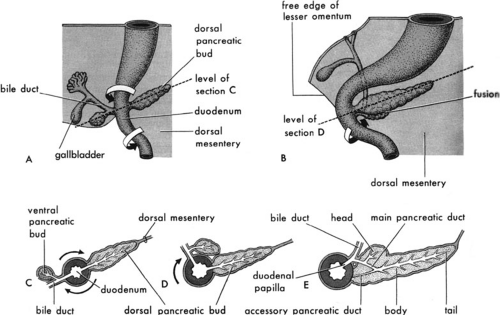
The human pancreas is of endodermal origin and is first detectable by the fifth week of gestation, originating as paired evaginations of the foregut (1). The dorsal pancreas arises as a diverticulum from the dorsum of the foregut just distal to the stomach. The ventral pancreatic bud actually arises from the biliary diverticulum. Together, the biliary and ventral pancreatic buds swing around the dorsal aspect of the foregut during gut rotation, and the ventral pancreatic bud eventually gives rise to the head of the pancreas, uncinate process, and the proximal portion of the main pancreatic duct. The dorsal pancreatic bud will give rise to the neck, the body, and the tail of the pancreas, as well as to the minor duct (duct of Santorini) and minor papilla, and the continuation of the main duct (duct of Wirsung) into the body and tail. The main pancreatic duct, along with the common bile duct, drains into the duodenum at the ampulla of Vater, usually as a single common channel (Fig. 44-1).
Formal fusion of the parenchyma of the two pancreatic buds is essentially complete by the seventh week of gestation; however, fusion of the ducts from each bud, to form the main pancreatic duct, is delayed until the perinatal period (2). The endocrine component of the pancreas, eventually contained within the islets of Langerhans, starts to differentiate before formation of the pancreatic buds in the area of the foregut from which the pancreas will arise (3). The islets make up 10% of the pancreas during fetal life, but that percentage decreases to less than 1% in the adult. Fetal pancreatic islets may play an important role in regulating glucose homeostasis late in gestation. Pancreatic acinar tissue begins to form at approximately 12 to 15 weeks, and later begins to accumulate zymogen granules characteristic of acinar cells. These fetal acinar cells do not secrete digestive enzymes until after birth (1).
ANATOMY
The pancreas is retroperitoneal and runs obliquely upward from right to left. The head of the pancreas lies within the C loop of the duodenum, and lies just anterior to the body of the second lumbar vertebra and aorta. The uncinate process projects out from the posteromedial portion of the head, and behind the superior mesenteric artery and vein. The neck of the pancreas is defined as the portion of the pancreas anterior to these vessels (4) The body and tail are to the left of these vessels. The body and tail are closely adjacent to the posterior wall of the stomach and to the spleen.
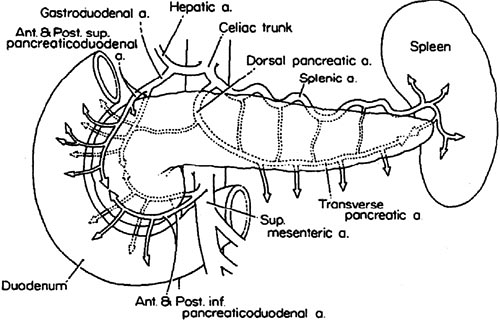
The major arterial blood supply to the pancreas is from the celiac and superior mesenteric arteries, branches of which form the pancreaticoduodenal arcades. The superior arcades are formed from the gastroduodenal artery and the inferior arcades from the superior mesenteric artery. Each arcade has anterior and posterior branches. It is important to note that the pancreaticoduodenal arcades supply both the pancreatic head and the duodenum. The body and tail of the pancreas are supplied by branches of the splenic artery, which runs along the posterosuperior surface of the pancreas toward the spleen (Fig. 44-2). In general, the venous drainage of the pancreas follows the arterial blood supply, with ultimate drainage into the portal vein (5).
CONGENITAL ANOMALIES
Ectopic pancreatic rests are congenital anomalies found in the gastrointestinal tract or in adjacent structures in less than 2% of cases from autopsy series. Ectopic pancreas is most frequently located in the duodenum, the stomach, or the jejunum (6). Ectopic pancreas is the most common anomaly of the gastric antrum, and cases of gastric outlet obstruction caused by ectopic pancreas have been reported (7,8). The origin of ectopic pancreatic rests is essentially unknown, but may be due to aberrant epithelial–mesenchymal interactions leading to the transdifferentiation of heterotopic embryonic epithelium into pancreatic epithelium. More recent work has demonstrated that defects in the hedgehog family of signaling molecules, which normally antagonize pancreas development, may be
permissive for the development of ectopic pancreatic tissue (9,10). In general, ectopic pancreas is usually asymptomatic and is discovered incidentally at laparotomy. Of those patients with symptoms, the lesions are usually found in the stomach or duodenum, with the most common complaint being epigastric pain (6). Rarely, an ectopic pancreatic rest produces obstruction and bleeding. Although controversial, incidental ectopic pancreatic rests should probably be excised (unless the excision would entail significant risk of morbidity).
permissive for the development of ectopic pancreatic tissue (9,10). In general, ectopic pancreas is usually asymptomatic and is discovered incidentally at laparotomy. Of those patients with symptoms, the lesions are usually found in the stomach or duodenum, with the most common complaint being epigastric pain (6). Rarely, an ectopic pancreatic rest produces obstruction and bleeding. Although controversial, incidental ectopic pancreatic rests should probably be excised (unless the excision would entail significant risk of morbidity).
Annular pancreasis believed to be due to errors in rotation of the ventral pancreatic bud around the posterior aspect of the duodenal anlage. The result is an abnormal embryologic connection between the ventral and dorsal anlage of the pancreas, which often is associated with an obstruction or atresia of the duodenum. As seen in ectopic pancreatic rests, abnormal endodermal expression patterns of sonic hedgehog, a potent intercellular signaling protein that demarcates a molecular boundary between pancreas and adjacent duodenum, may be responsible for development of annular pancreas (9). Anomalies associated with annular pancreas include duodenal atresia and stenosis (∼50%), intestinal malrotation, and trisomy 21 (20%) (11). The pancreatic tissue within the annular pancreas appears to have normal functioning acini, ducts, and islets of Langerhans (12,13). The primary symptomatology of annular pancreas results from duodenal obstruction. Generally, the patients can present with bilious vomiting if the obstruction is distal to the ampulla of Vater. The classic radiographic finding is the “double bubble” sign (14). Therapy involves surgical bypass of the lesion with a side-to-side duodenoduodenostomy, or possibly duodenojejunostomy as a second alternative.
Pancreas divisum is an anomaly of the pancreatic ducts and is caused by the lack of fusion of the ventral and dorsal pancreatic ducts during embryologic development. It is the most common pancreatic anomaly and is found in 5% to 10% of the population. The clinical significance of pancreas divisum remains unclear; however, the associated relative obstruction at the minor papilla has been implicated as a potential cause of pancreatitis, although this point is an area of controversy (15,16,17). In patients with this anomaly, the minor duct of Santorini drains the majority of the exocrine secretions from the body and tail of the pancreas. Sphincteroplasty of the minor papilla has led to improvement in the symptoms of pancreatitis in many of these patients, providing strong evidence for the role of minor papillae stenosis as the cause of pancreatitis in certain patients with pancreas divisum (17). An alternative to surgical sphincteroplasty is endoscopic therapy, with stenting and/or sphincterotomy. This endoscopic procedure should only be performed by a skilled endoscopist with experience in cannulation of the smaller ducts encountered in children (18).
PANCREATITIS
Acute Pancreatitis
Acute pancreatitis is due to inflammation of the pancreas that may vary in intensity from mild, local inflammation to fulminant necrotizing pancreatitis with subsequent systemic inflammatory response resulting in end-organ injury and even death. Tissue injury is due to direct enzymatic injury with secondary inflammation. Involvement of the gland can progress with microvascular ischemia. Generation of free radicals and peroxides can further perpetuate and extend the injury. Acute pancreatitis is believed to only induce reversible changes in morphology and function, as opposed to the irreversible changes seen in chronic pancreatitis.
The possible etiologies of acute pancreatitis in children include trauma, biliary tract disease, choledochal cysts, ductal anomalies, drugs, metabolic derangements, and infections (Table 44-1). The etiology is unknown in more than 30% of cases.
The pathogenesis of acute pancreatitis is poorly understood. Several mechanisms may initiate pancreatic inflammation, including increased permeability of the pancreatic duct, overstimulation of the gland, obstruction to pancreatic flow, overdistention of the pancreatic ductal system, toxin exposure, and metabolic abnormalities (15). The possible causes of inappropriate activation of pancreatic enzymes include (1) reflux of duodenal enterokinase into the pancreas to activate trypsin, which then inappropriately activates other proenzymes in the pancreas; (2) ductal obstruction with extravasation of enzyme-rich ductal fluid into the parenchyma of the pancreas; or (3) fusion of lysosomes with zymogen granules inside acinar cells to allow lysosomal enzyme activation of the proenzymes. Once activated, elastase, phospholipase, and superoxide free radicals are believed to be the principal mediators of tissue damage.
Traumatic pancreatitis is relatively common in the pediatric population. The pancreas is the fourth most commonly injured intraabdominal solid organ in children with blunt trauma (19). The mechanism of injury usually involves anterior compressive forces applied to the pancreas,
which lies fixed against the vertebral column. The classic mechanism of injury in children is the blunt impact of a bicycle handlebar to the upper abdomen (20). Biliary tract stone disease, although less common in children, may lead to pancreatitis from transient pancreatic duct obstruction with or without bile reflux. Data from the adult experience suggests that removal of impacted stones in severe gallstone-induced pancreatitis is best accomplished by endoscopy with sphincterotomy, and not through surgery (15). Choledochal cysts may produce pancreatitis through transient pancreatic duct compression or bile reflux resulting from a long common biliary-pancreatic duct within the head of the pancreas. More than 85 different drugs have been reported to cause acute pancreatitis. The drugs with the strongest association include azathioprine, mercaptopurine, didanosine, corticosteroids, and valproic acid (21,22,23). Systemic illnesses and metabolic conditions may cause pancreatitis, such as cystic fibrosis, Reye’s syndrome, Kawasaki’s disease, hyperlipidemias, and hypercalcemia. Infections with viruses such as coxsackievirus and rotavirus join a long list of other organisms responsible for causing pancreatitis (23).
which lies fixed against the vertebral column. The classic mechanism of injury in children is the blunt impact of a bicycle handlebar to the upper abdomen (20). Biliary tract stone disease, although less common in children, may lead to pancreatitis from transient pancreatic duct obstruction with or without bile reflux. Data from the adult experience suggests that removal of impacted stones in severe gallstone-induced pancreatitis is best accomplished by endoscopy with sphincterotomy, and not through surgery (15). Choledochal cysts may produce pancreatitis through transient pancreatic duct compression or bile reflux resulting from a long common biliary-pancreatic duct within the head of the pancreas. More than 85 different drugs have been reported to cause acute pancreatitis. The drugs with the strongest association include azathioprine, mercaptopurine, didanosine, corticosteroids, and valproic acid (21,22,23). Systemic illnesses and metabolic conditions may cause pancreatitis, such as cystic fibrosis, Reye’s syndrome, Kawasaki’s disease, hyperlipidemias, and hypercalcemia. Infections with viruses such as coxsackievirus and rotavirus join a long list of other organisms responsible for causing pancreatitis (23).
TABLE 44-1 Causes of Acute Pancreatitis. | |
|---|---|
|
The clinical presentation of acute pancreatitis usually involves sudden onset of midepigastric pain with radiation to the back, vomiting, and low-grade fever. The abdomen is diffusely tender with signs of peritonitis, and there is abdominal distention with a paucity of bowel sounds. In severe cases, signs of hypovolemic shock may be present. In cases of necrotizing or hemorrhagic pancreatitis, hemorrhage may dissect from the pancreas along tissue planes, presenting as ecchymosis either in the flanks (Grey-Turner sign) or around the umbilicus (Cullen’s sign). Measurements of serum amylase are helpful, although a normal level does not preclude the diagnosis of acute pancreatitis. Also, the degree of amylase elevation does not correlate well with the severity of the pancreatitis. Amylase can be measured in the urine, but similar to the renal handling of glucose, tubular reabsorption prevents amylase spilling into the urine until significant hyperamylasemia is present (24). It is important to note that hyperamylasemia or hyperamylasuria may be caused by conditions other than pancreatitis, such as salivary trauma, renal failure, macroamylasemia, small bowel pathology including perforation, ischemia, necrosis, or inflammation. The serum lipase level is less sensitive, but more specific for pancreatitis, and levels remain elevated for longer periods of time than amylase levels. An elevated lipase level is particularly helpful for distinguishing pancreatic trauma from other sites of trauma (25,26).
Imaging studies play an important role in the evaluation of patients with acute pancreatitis. Plain abdominal radiographs may reveal an isolated dilated loop of intestine, typically in the upper abdomen near the inflamed pancreas, referred to as a sentinel loop. Local spasm of the transverse colon, with proximal dilatation is known as the colon cut-off sign. Occasionally, calcifications may be seen within the region of the pancreas, which suggests chronic pancreatitis. A chest roentgenogram should also be obtained in all patients to evaluate the possibility of pleural effusion and pulmonary edema.
Abdominal ultrasound (US) is a noninvasive test, but resolution is poor when there is overlying bowel gas interference. In addition, US findings are not reliable in determining the severity of pancreatic inflammation (27). US is most useful in demonstrating gallstones as a possible cause of pancreatitis, and in monitoring for improvement in edema or peripancreatic fluid collections (28).
Abdominal computed tomography (CT) is probably the best modality for evaluation of pancreatitis, allowing detection of pancreatic inflammation as well as abnormal extrapancreatic fluid collections (29). CT provides better resolution than US in determining the size of the pancreas and the degree of edema. By using a rapid infusion of contrast with concomitant rapid scanning in fine cuts through the pancreas, a precise assessment of the percentage of the pancreas that is receiving less than normal blood flow (or no blood flow) can be made. This degree of ischemia correlates with prognosis. Another advantage of CT scan is that it may be used in conjunction with interventional procedures for the diagnosis or drainage of peripancreatic fluid collections (30).
Endoscopic retrograde cholangiopancreatography (ERCP) is being used with increasing frequency in the pediatric and even neonatal population. More recent reports suggest that complication rates in children are higher than in the adult population (31). In contrast to adults, ERCP may not be indicated for the initial diagnosis of acute pancreatitis in children, but may be helpful in complicated cases of acute pancreatitis. In addition, ERCP may play a role in evaluating the integrity of the pancreatic ducts in cases of pancreatic trauma, and in cases of refractory biliary pancreatitis, where a stone may be impacted at the ampulla of Vater.
Magnetic resonance cholangiopancreatography (MRCP) is a relatively new, noninvasive technique for evaluating the biliary tree and the pancreatic duct. This technology provides the ability to delineate ductal anatomy, but has a lower complication rate than ERCP, is less expensive, and requires no radiation or contrast injection. Although MRCP does not allow for therapeutic interventions, it may be helpful in directing the types of intervention that may be required [ERCP, percutaneous transhepatic cholangiography (PTC), or surgery] (32). The disadvantages of MRCP are that it tends to overestimate the stenosis of the main pancreatic duct, and underestimate the degree of dilatation of the branches and filling defects of the pancreatic duct in patients with pancreatitis. MRCP is now becoming the initial imaging study of choice in the evaluation of pancreatic ductal anatomy in children with unexplained or recurrent pancreatitis (18).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree