Organization of Care and Quality in the NICU
Richard J. Powers
Carolyn Lund
▪ INTRODUCTION
The delivery of care in the neonatal intensive care unit (NICU) is a complex process involving numerous disciplines and personnel. Neonatologists, nurses, respiratory therapists, social workers, developmental care specialists, pharmacists, clinical dieticians, and occupational and physical therapists have roles to play in planning, implementing, and evaluating care for infants and their families in the NICU. The day-to-day management is important to the overall organization and keeps operations in motion. However, to continually improve practice and reduce medical errors, a system of continuous quality improvement (CQI) is needed.
In recent decades, medicine has witnessed a rapid expansion of knowledge and technology. This expansion has occurred in parallel with financial pressures brought on by relentless increases in per capita costs of health care in the United States and limitations in financial resources available to the health care system. These forces are especially applicable to intensive care subspecialties such as neonatology on which a significant amount of research and technology is focused and for which delivery of care can be extremely costly.
The volume of available research for practitioners to review and integrate into practice has increased exponentially in recent years. In 1966, approximately 100 articles were published annually in all fields of medicine from randomized controlled trials (RCTs), and current estimates are more than 10,000 RCTs published annually (1). In pediatrics alone, there are 191 specialty-specific journals with more than 22,000 pediatric articles published in 2010 (2). In addition to the volume of published research, the health care industry has been challenged to provide more accountability in light of public information such as the Institute of Medicine (IOM) report estimating 98,000 preventable hospital deaths per year in the United States and other reports of excessive errors, medical errors, and adverse events (3,4,5).
The field of neonatology is especially vulnerable to the occurrence of adverse events due to the rapid development of technology with limited evidence supporting widespread adoption (6). The small size, critical medical conditions, and longer length of stay of NICU patients compound their risk of exposure to complications. The Harvard Medical Practice Study reported that 1.2% to 1.4% of NICU patients experience a medical error during their hospitalization (7,8). Ligi reported the incidence of iatrogenic events in the NICU at 25.6 per 1,000 patient-days and that over one-third of the iatrogenic events in the NICU are preventable (9).
In the face of this mounting information on clinical efficacy from RCTs of various treatments, the rapid infusion of technology, and the high prevalence of preventable complications, neonatal practitioners and institutions face major challenges. Neonatal practitioners in health care organizations need to efficiently evaluate new interventions and adopt the most compelling ones in a timely manner, in order to provide optimal patient care and avoid preventable complications. It is through the principles of quality improvement (QI), along with organizational adaptability, that continuous integration of research, technology, and improved patient care outcomes is accomplished.
▪ BACKGROUND OF QUALITY IMPROVEMENT
Quality of care is defined by the IOM as “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge.” (10) This definition, first proposed in 1990, is now widely accepted and is still considered the best definition of health care quality today. The concept of “health services for individuals and populations” is especially important in neonatology, where evaluation of treatment is often determined by population data such as infant and neonatal mortality rates or the incidence of neurologic deficits among a specific subgroup such as extremely low-birth-weight survivors.
The IOM definition also emphasizes that quality care “increases the likelihood” of beneficial outcomes, a reminder that quality is not merely the achievement of positive outcomes. Poor outcomes occur despite excellent care because diseases vary in severity and can defeat even the best efforts. Conversely, patients may do well despite poor quality of care. Assessing quality thus requires attention to both processes and outcomes of care. The last part of the definition of quality, “consistent with current professional knowledge,” highlights the dynamic and evolving body of knowledge available to health care professionals and the need to revise and update measures of quality as new interventions become standards of care.
Problems in quality of health care can be classified in three categories: underuse, overuse, and misuse (11). Underuse is the failure to provide a health care service when it has a significant likelihood of producing a favorable outcome, for example, failure to provide surfactant in a timely manner after the delivery of an extremely low-birth-weight infant with respiratory distress syndrome. Overuse occurs when a health care service is provided despite the fact that its potential for harm exceeds its possible benefit. The widespread use of postnatal steroids for chronic lung disease, popular in the 1990s, is an example of overuse in neonatology. Misuse occurs when a preventable complication arises during administration of an appropriately selected treatment. Misuse includes many of the common medical errors that occur during hospitalization or other health care encounters.
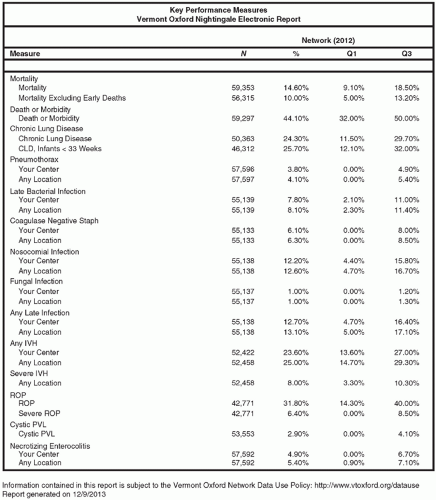
Medical errors have been extensively discussed (3), driving numerous initiatives by governmental and regulatory agencies aimed at understanding the human and systems factors that contribute to errors (see also Chapter 9). External reporting systems that collect information on adverse events and errors are important in the reduction of future errors by alerting practitioners to new hazards, using the experience of individual hospitals applying new methods to prevent errors and revealing trends that require attention (12). In neonatology, medical errors have been collated and classified as part of an anonymous error-reporting project in conjunction with the NIC/Q Quality Improvement Collaborative of the Vermont Oxford Network (Fig. 3.1) (13).
Regulatory agencies, in conjunction with federal and state governments, have traditionally been charged with the task of motivating health care professionals and organizations to maintain and improve quality. The Joint Commission on Accreditation of Healthcare Organizations (JCAHO), formed in 1951, initially developed standards for hospitals and evaluated compliance to these standards, hypothesizing that compliance with these standards would correlate with quality care and positive outcomes for patients in hospitals. In accreditation, quality is evaluated by monitoring adherence to accepted standards and measuring outcomes. Standards used by accreditation organizations are derived from a variety of sources, including government (via regulatory agencies at both the federal and state levels), as well as professional and community-based standards of practice.
Regulation is, for the most part, successful in establishing minimal standards of performance and is an important means of protecting the public from egregiously poor providers. It has, however, numerous limitations. Standards are difficult to enforce uniformly, and regulation tends to be inflexible with difficulty in adapting quickly as knowledge changes. Regulation also fails to stimulate organizations to integrate new technologies or developments and does not motivate them to continuously improve. CQI can supplement the deficiencies of regulation alone, while providing an impetus for individuals and organizations to strive for the highest quality of care.
▪ CONTINUOUS QUALITY IMPROVEMENT
The health care industry has learned from the industrial sector that CQI is an effective system to reduce errors. It motivates good performers to excel, emphasizes identification of potentially successful change opportunities, and facilitates change implementation throughout all levels of the organization. CQI provides the framework for organizations to keep abreast of current knowledge and innovations, identify appropriate changes, and implement them in a timely manner. There are three components to CQI: measurement, benchmarking, and action.
CQI Measurement
An important fundamental in bringing about change is the creation of urgency, a motivational force leading to alignment of goals within an organization. This provides an answer to the question of “what do we need to change?” The basic element in identifying the priorities for QI is the acquisition of data. Data acquisition drives information, which in turn drives action. Over the past three decades, numerous systems of quality measurement have been developed, encompassing the areas of outcomes, processes, and patient satisfaction.
Outcome measures represent the most objective and often the most meaningful data for health care organizations. When applied to populations, outcome measures provide essential feedback to leaders charged with resource allocation, managers charged with developing successful and efficient organizations, and individual health care providers.
Due to variability in disease severity among patients from different socioeconomic and cultural backgrounds, as well as differences in the type of patients cared for in highly specialized tertiary centers compared to community health facilities, data based on outcomes alone can be inaccurate or misleading. Process measures are also important in evaluating overall quality. Measures of process are needed to determine that accepted standards of care are being met regardless of good or bad outcomes.
Several mandatory measures are required of hospitals in order to maintain accreditation and receive reimbursement. These include the JCAHO Core Measure Sets addressing care in the areas of acute myocardial infarction, heart failure, pneumonia, and surgical and perinatal care. The perinatal care measure set became a requirement for all hospitals with greater than 1,100 births per year in January 2014. There are five perinatal care measures: elective delivery, cesarean section, antenatal steroids, health care-associated bloodstream infections in newborns, and exclusive breast-feeding. Bloodstream infections, essentially central line-associated bloodstream infections (CLABSIs), are one of the primary measures used in NICU quality improvement work and are tracked in most NICU databases using various definitions. The Centers for Disease Control and Prevention (CDC) Neonatal Health Services Network (NHSN) definition is the most commonly used definition for CLABSIs (14).
A third category of measurement in assessing quality of care is patient and family satisfaction. This is the result of applying traditional marketing techniques to the health care industry, and it has accompanied the adoption of CQI principles from industry to health care. It is also a natural result of a larger movement throughout health care that recognizes the autonomy and accountability of the patient and family. As the health care community expands its expectations that patients and their families take on a larger part of the responsibility to maintain their own health and wellness, the feedback obtained from patients and families regarding their interaction with the health care system is crucial. Table 3.1 shows examples of neonatology quality measures in the three areas of outcomes, processes, and patient satisfaction.
Hospitals have collected information on patient and family satisfaction using internal tools or through various outside vendors. More recently, the Center for Medicare and Medicaid Services of the U.S. Department of Health and Human Services has required the administration of a national standardized survey of patients’ perspectives of hospital care. This survey, the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS), was designed to provide a national standard for collecting and publicly reporting information about patient experience that allows valid comparisons to be made across hospitals locally, regionally, and nationally. The HCAHPS survey provides limited information on the NICU family experience, thus many NICUs still rely on other standardized tools for obtaining feedback and benchmarking outcomes related to the family experiences during the NICU hospitalization of their newborn (15,16).
TABLE 3.1 Typical Quality Improvement Measures | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
CQI Benchmarking
Use of Comparative Databases
The second step in the process of CQI is benchmarking of outcomes. Opportunities for benchmarking are numerous due to the recognition of the contribution it brings to CQI. A number of regional, national, and international databases have been organized in neonatology, providing benchmarking opportunities through voluntary participation and confidential reporting of individual center outcomes.
One of the first neonatology databases, and currently the largest, is the Vermont-Oxford Neonatal (VON) Network. Initiated in 1990 with 36 hospitals, this network has grown to 750 centers (17,18,19). The network includes data on more than 53,000 very-low-birth-weight (VLBW, ≤1,500 g) infants each year from U.S. and international participating institutions. The network also maintains an expanded database of 241 hospitals reporting on over 106,000 infants greater than 1,500 g.
In the VON Network, centers report outcomes, including survival and length of stay for infants admitted to the NICU. They also report incidence of chronic lung disease and complications, nosocomial infection, pneumothorax, necrotizing enterocolitis, intraventricular hemorrhage, retinopathy of prematurity, and other conditions. All participating centers receive a confidential annual report showing their performance compared with the database as a whole. Each center can evaluate how they rank with all other centers and with centers that are grouped in similar categories by number and type of NICU admissions. In the VON Network, all of the variables are reported in aggregate form showing the mean incidence rate and highest and lowest quartile of each measure. The mortality rate and length of stay for each center are also adjusted for patient acuity.
Other databases have been formed at regional and national levels. The National Institute of Child Health and Human Development Neonatal Research Network provides a venue for participating institutions to submit outcome measures; their aggregate data have been published to serve as a reference for other centers to compare their performances. These include general survival and complication rates in VLBW infants (20) and rates of neurologic abnormalities
(21,22). The Children’s Hospitals Neonatal Database (CHND) contains demographic, treatment, and outcome data on NICU patients from more than 25 U.S. children’s hospital NICUs. This database was designed to develop benchmarking data for the care of infants who are typically cared for in Children’s Hospitals, particularly infants requiring surgical care or management by multiple pediatric sub-specialists. Examples include infants with surgical anomalies, such as gastroschisis, tracheoesophageal fistula, and necrotizing enterocolitis, or those with severe bronchopulmonary dysplasia (23).
(21,22). The Children’s Hospitals Neonatal Database (CHND) contains demographic, treatment, and outcome data on NICU patients from more than 25 U.S. children’s hospital NICUs. This database was designed to develop benchmarking data for the care of infants who are typically cared for in Children’s Hospitals, particularly infants requiring surgical care or management by multiple pediatric sub-specialists. Examples include infants with surgical anomalies, such as gastroschisis, tracheoesophageal fistula, and necrotizing enterocolitis, or those with severe bronchopulmonary dysplasia (23).
The Pediatrix Medical Group Clinical Data Warehouse (CDW) captures aggregate data from over 20,000 VLBW infants annually through the integration of the electronic medical record used for bedside documentation by neonatal clinicians. The passive acquisition of data in the CDW allows a much broader scope of analysis, since the variables are not limited to preselected data sets (24).
The passive acquisition of data has been made possible through development of functional electronic medical record systems that are designed to store data as it is being entered, rather than the retrospective extraction of data from the written or transcribed elements of the patient chart. The inherent efficiencies of passive acquisition in real time, with the elimination of retrospective chart review and additional steps of data entry, can lead to a more comprehensive database. A major concern about passively acquired data is the challenge of maintaining data accuracy when compared to retrospective data entry following rigorous criteria for defining clinical variables.
The Canadian Neonatal Network receives input for all level III NICUs in Canada. It has also published reports tracking overall outcomes and complications in infants of all gestational ages (25). In the United States, numerous statewide databases have been formed by neonatology organizations to promote local participation in benchmarking and collaborative QI projects. The California Perinatal Quality Care Collaborative (CPQCC) was the first of these to be organized and has been the model for many other states (26).
Variability in NICU Outcomes
Variability in neonatal outcomes becomes apparent when data from multiple NICUs are analyzed in comparative databases. Annual VON reports provide the distribution of outcomes among centers by ranking the data and calculating the 25th and 75th percentiles for mean incidence of the given outcome from each center. Percentile ranking of mean values from individual centers represents a simple and effective means of illustrating one center’s ranking among the entire sample of participants. Figure 3.1 shows recent data from VON, illustrating this methodology for selected outcomes (27).
Investigators also report variation in outcomes as part of multicenter prospective interventional trials or retrospectively as multicenter independent research. Brodie and associates (28) studied nosocomial bloodstream infections in VLBW infants in six NICUs in the Boston area from 1994 to 1996; mean incidence of infections was 19.1% for the whole group but varied from 8.5% to 42% among the six units. After adjusting for patient- and treatment-related variables, significant variation persisted. Variation in blood transfusions has been studied, showing a mean total transfused volume ranging from 95.5 (highest) to 35.0 mL/kg (lowest) (29). Avery and associates (30) described the variability in incidence of chronic lung disease among eight units surveyed. Later, an in-depth review of the practices related to respiratory support for infants with respiratory distress syndrome was undertaken at the eight centers, triggering the study and dissemination of a number of innovations in respiratory care practices from the unit reporting the best outcome. Wide variation in outcomes among centers is often found when centers participate in comparative outcome studies. Even when the data have been adjusted for confounding risk factors, marked variability still exists in many cases. Explanations for this persistent variation include differences in case mix, data quality, and case finding. However, the final and most important factor is often variation in effectiveness of clinical practice.
One of the major benefits of participating in the comparative analyses of outcome measures lies in the understanding that changing clinical care practices truly can influence their outcomes. In most cases, individual units find outcomes in the lowest quartile for only a few variables of their data set, with the majority falling within the interquartile range (25th to 75th percentile) or even exceeding the 75th percentile. The lowest quartile outcomes provide target areas to which focused improvement efforts can be directed. Furthermore, centers in the database that report better outcomes can be used as resources to identify practices that may benefit centers in the lowest quartile.
Published data can also be used as a benchmark when concurrent data are not available. It is important to review the methodology and data definitions in the published benchmark paper to allow for consistency in data acquisition before any extrinsic comparison can be made. An example is the CLABSI rate published by the National Healthcare Safety Network, which is used by many NICUs to analyze their infection prevalence (14). This benchmark definition, used as the gold standard for most public reporting systems, has been periodically modified to provide better precision in generating comparative data.
Evidence-Based Medicine
Evidence-based medicine is defined as “the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients (31).” In the context of CQI, the definition is expanded beyond the individual patient to decisions regarding institutional policies in the care of multiple patients with similar diagnoses. In both applications, the principles are the same: an answerable clinical question is formulated, the best evidence is located, and the evidence is critically appraised. These steps are essential whether answering a question regarding treatment for a VLBW infant with a patent ductus arteriosus or identifying best practices to be implemented in the management of certain VLBW admissions who meet criteria for the diagnosis of patent ductus arteriosus.
Randomized Controlled Trials
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree