Obstetric Hemorrhage
Carol J. Harvey
Gary A. Dildy
Obstetric hemorrhage remains one of the top three causes of maternal mortality in industrialized and developing nations.1 The overwhelming majority of maternal deaths occur in developing nations. Data from the World Health Organization estimate that the global maternal death rate exceeds 536,000 per annum. Data also indicate that 25 percent of the total number of maternal deaths were because of severe bleeding.2 It is important to note that obstetric hemorrhage is a potentially life-threatening condition not limited to any specific geographic boundary or patient population.
Berg and colleagues estimate that 90 percent of maternal deaths secondary to postpartum hemorrhage (PPH) are preventable.3 Clark and colleagues, in their report of data from a series of 95 maternal deaths in a large U.S. health care organization, estimate that 73 percent of deaths due to PPH were preventable.3,4 Prevention of adverse outcome is dependent upon recognition of risk factors, timely identification of abnormal bleeding, and prompt initiation of appropriate clinical management. Prompt management requires detection of abnormal bleeding, laboratory assessment, pharmacologic intervention, and in some cases blood component therapy and surgical intervention.
The American College of Obstetricians and Gynecologists’ (ACOG) position on PPH is quite clear: “All obstetric units and practitioners must have the facilities, personnel, and equipment in place to manage this emergency properly.”5 Thus, all practitioners of obstetrics should be prepared to identify and appropriately respond to this potentially life-threatening complication of pregnancy.
The purpose of this chapter is to address the following concepts: normal physiology of pregnancy with regard to blood volume expansion and peripartum blood loss, etiologies of hemorrhage, clinical estimation of blood loss, clinical management of hemorrhage, and blood component therapy. The inherent need for collaboration is reinforced. Clinical case excerpts are presented to reinforce application of concepts to practice.
Physiology of Pregnancy and Peripartum Blood Loss
During pregnancy the maternal plasma volume expands by approximately 42 percent, while red blood cell (RBC) volume increases 24 percent, increasing overall blood volume while at the same time producing the so-called “physiologic anemia of pregnancy” phenomenon.6 This net increase in blood volume is generally sufficient to compensate for blood loss that normally occurs during the third stage of labor when the placenta detaches. Women with hypertensive disorders of pregnancy have diminished maternal plasma volume expansion; as such, they are not only less able to tolerate hemorrhage, but are also at greater risk for hemorrhage.
Average blood losses at spontaneous vaginal delivery, Cesarean delivery, and elective Cesarean hysterectomy are approximately 500 mL, 1000 mL, and 1500 mL, respectively.7 Expressed as a percentage of total blood volume, average blood losses at spontaneous vaginal delivery, Cesarean delivery, and elective Cesarean hysterectomy are approximately 10 percent, 25 percent, and 33 percent, respectively. However, when emergency Cesarean hysterectomy is performed, average blood loss has been estimated to be 3500 mL, which represents more than 75 percent of the total maternal blood volume at term.8 It has been estimated that women who undergo operative vaginal delivery (forceps and/or vacuum) lose as much blood as those who undergo Cesarean delivery.9 It has also been shown that the degree of any perineal laceration correlates positively with the degree of blood loss, such that women with third- or fourth-degree lacerations may lose as much blood as women who undergo Cesarean delivery.9
Postpartum hemorrhage has been traditionally defined as an estimated blood loss in excess of 500 mL. However this definition is somewhat arbitrary, and as noted above, half of all women lose at least 500 mL at spontaneous vaginal delivery. This discrepancy can be partly explained by the fact that blood loss at delivery is usually underestimated.10 The incidence of PPH, based on a definition of a 10 percent drop in hemoglobin and/or hematocrit, or the need for a blood transfusion, is approximately 4 percent of vaginal deliveries and 6 percent of Cesarean deliveries.11 Thus, approximately 1 in 20 women will experience PPH.
PPH is classified as primary when the bleeding occurs in the first 24 hours after birth and secondary if excessive blood loss from the vagina begins more than 24 hours postpartum and prior to 6 weeks following delivery.
There is no consensus with respect to the definition of massive transfusion in the obstetric patient. For the purpose of this chapter, massive transfusion is defined as replacement of the patient’s total blood volume within 24 hours. For actively bleeding patients, massive transfusion is defined as transfusion of 10 or more units of packed red blood cells (PRBCs) within 24 hours. Massive transfusion most commonly occurs in patients with significant traumatic injuries, gastrointestinal bleeding, or PPH.12 Frequently, patients with obstetric hemorrhage and coagulopathy will require 10 or more units of PRBCs in 2 hours or less.12 This patient population is at significant risk for exsanguination, vascular collapse, and death.
Table 15.1 Etiologies of Obstetric Hemorrhage | ||||||
|---|---|---|---|---|---|---|
|
Etiologies of Obstetric Hemorrhage
Obstetric hemorrhage is a clinical sign, not a diagnosis. In order to provide proper therapy, a correct diagnosis must be made. A thorough discussion of all potential etiologies for obstetric hemorrhage is beyond the scope of this chapter. Although some concepts presented in this chapter apply to any patient with obstetric hemorrhage, PPH is a focus of the text. For example, etiologies of PPH are listed in Table 15-1. The most common etiology of PPH is uterine atony, followed by retained placenta and lower genital tract lacerations. This list is not exhaustive; thus, hemorrhage should be considered even when the patient’s uterus is firm and visual inspection of the lower genital tract is negative. Continued vaginal bleeding in the presence of a firmly contracted uterus should prompt the delivery provider to specifically assess for cervical and/or vaginal lacerations.
Patients at Risk for Obstetric Hemorrhage
Risk factors for hemorrhage are prevalent in the general obstetric population, and some women who develop hemorrhage will have no obvious risk factors. Since obstetric hemorrhage is relatively common, clinicians should be well versed and prepared to care for patients who develop this complication. Prolonged labor, uterine over-distention (e.g., large baby, multiple gestation, polyhydramnios), and intrapartum infection are all associated with an increased risk for PPH. Additional risk factors for PPH are presented in Table 15-2. It should be appreciated that some antenatal conditions that involve abnormal placentation or maternal complications (e.g., placenta previa, placenta percreta, placental abruption) may result in obstetric hemorrhage with increased risk for maternal–fetal morbidity and mortality. In a large series of pregnancies complicated by placenta percreta, a 7 percent maternal mortality rate was reported; half of these deaths occurred in cases where percreta was suspected prenatally.13
Table 15.2 Risk Factors for Postpartum Hemorrhage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
There are different levels of pre-delivery preparations applied to these conditions. For the woman who has a protracted labor with a large baby and suspected intrapartum chorioamnionitis, preparation would include establishment of adequate intravenous access, type and crossmatch of PRBCs and fresh frozen plasma (FFP), and arrangement for the immediate availability of uterotonic agents. For the woman with suspected abnormal placentation (e.g., percreta), pre-delivery preparation becomes more intricate and may
include establishment of central venous access with insertion of a catheter type approved for high-pressure infusions, placement of an intra-arterial catheter for continuous blood pressure assessment and obtaining samples for arterial blood gas analysis, femoral intra-arterial access for large vessel embolization, preparation of a cell salvage device, availability of a Level I or rapid-volume infuser, crossmatch for a larger number and variety of blood components, active patient warming devices, multidisciplinary consultation, and preparation for special approaches to hysterectomy.14
include establishment of central venous access with insertion of a catheter type approved for high-pressure infusions, placement of an intra-arterial catheter for continuous blood pressure assessment and obtaining samples for arterial blood gas analysis, femoral intra-arterial access for large vessel embolization, preparation of a cell salvage device, availability of a Level I or rapid-volume infuser, crossmatch for a larger number and variety of blood components, active patient warming devices, multidisciplinary consultation, and preparation for special approaches to hysterectomy.14
Assessment of Blood Loss
Underestimation of blood loss is common. This can lead to delay in treatment and subsequent increased risk for morbidity and mortality. The triennial reports of the Confidential Enquiries into Maternal Deaths showed that many maternal deaths secondary to hemorrhage in the United Kingdom are attributed to delayed diagnosis and delayed blood component therapy.15 Studies assessing estimation of blood loss have shown that accuracy is not dependent upon the clinician’s age or years of experience, and that improvement in a clinician’s ability to accurately estimate blood loss can be achieved using simple educational methods.10
In everyday clinical practice, blood loss estimation can be performed by subjective visual means as well as by objective methods involving measurement of blood volume collected in containers or weighing surgical materials such as laparotomy (lap) sponges. As a general rule, 1 mL of blood weighs approximately 1 gram; thus, a 75-gram lap sponge contains approximately 75 mL of blood (minus the weight of the lap sponge). Another useful parameter to remember is that a fully saturated standard 18-inch × 18-inch lap sponge holds approximately 100 mL of whole blood. Figure 15-1 illustrates the approximate amount of blood contained on saturated surgical laps and sponges.
The accurate estimation of blood loss is critical in planning interventions for the patient who is actively bleeding and can be done by multiple providers.
Shock
Shock is defined as inadequate oxygen delivery and tissue perfusion secondary to decreased intravascular volume; it may progress to cellular hypoxia, acidosis, organ system damage, and death.16 Shock is commonly classified by causative etiologies, which are listed in Table 15-3.
Table 15.3 Shock: Etiologies and Differential Diagnosis | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Because the body attempts to compensate for a decrease in intravascular volume by vasoconstriction and preferential shunting of arterial blood to the heart and brain, the onset of shock may not initially be accompanied by hypotension. Additionally, some obstetric patients may not demonstrate a compensatory increase in their heart rate during the early stages of shock, which may mislead the practitioner and complicate timely diagnosis and early treatment.
Pharmacologic Therapy for Uterine Atony
Initial management of uterine atony involves bi-manual uterine massage performed by the delivering provider and medical therapy to enhance uterine contractility. A detailed presentation of concepts related to pharmacologic adjuncts in the care of obstetric patients with selected complications is included in Chapter 6 of this text. Achievement of normal uterine tone may be augmented by draining the urinary bladder if it is over-distended. Pharmacologic therapy is summarized in Table 15-4 and consists of administration of oxytocin, ergot alkaloids, and/or prostaglandins. Providers should be trained in the estimation of blood loss and respond collaboratively to acquire uterotonic drugs, provide safe administration of the agents, and assess the woman’s hemodynamic status and response to therapy. Oxytocin is generally considered the first line of therapy, as it is commonly administered as a prophylactic measure and has few contraindications (e.g., the patient reports a known/suspected allergy). There is no consensus with respect to the order in which prostaglandins (E or F class) and ergot alkaloids should be administered. Ergot alkaloids should be avoided in hypertensive women, and the F-class prostaglandins should be avoided in women with reactive airway disease such as asthma.
Misoprostol (Cytotec) is a PGE1 analogue, originally marketed for the prevention and treatment of peptic ulcer disease, commonly used off-label in obstetrics because of its effect on uterine contractility. A thorough discussion of this pharmacologic agent is presented in Chapter 12 of this text. This drug is advantageous because it is inexpensive, light and heat stable, and has a long shelf life. Misoprostol is probably safe in the setting of hypertension, has been studied extensively in the routine management of the third stage of labor, and is rapidly absorbed via the oral, vaginal, and rectal routes. While not demonstrated to be superior to other drugs in the prevention of PPH, a small randomized clinical trial demonstrated that misoprostol (800 mcg per rectum) is superior to oxytocin and ergot alkaloids in the primary treatment of PPH.17 Further randomized controlled trials are required to identify the best drug combination, route, and dose for the treatment of PPH.18 In the presence of PPH, misoprostol should be administered rectally or orally, depending on the patient’s condition and potential need for surgery and general anesthesia.
General Management of Obstetric Hemorrhage
Prevention of hemorrhage-related complications includes early recognition of abnormal blood loss and early mobilization of resources. Careful assessment and interpretation of maternal vital signs are critical in patient care during active bleeding. Obstetric patients, however, may not
show signs and symptoms usually observed in nonpregnant patients with hemorrhage until approximately one-third of the woman’s entire blood volume is lost.
show signs and symptoms usually observed in nonpregnant patients with hemorrhage until approximately one-third of the woman’s entire blood volume is lost.
Table 15.4 Pharmacologic Management of PPH: Uterotonic Agents | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
Table 15.5 Classification of Hemorrhage Based on Clinical Assessment | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
In the setting of postpartum bleeding, vital signs usually trend such that pulse rate increases, blood pressure decreases, and pulse pressure decreases as blood loss progresses. This concept is presented in Table 15-5. Urine output also declines as hypovolemia worsens. Tachycardia may be compensatory or occur secondary to other influences such as infection and pain.
Irrespective of the cause, maternal tachycardia in the postpartum woman should alert the clinician to the possibility of hypovolemic shock, as the potential for serious blood loss may occur when the maternal pulse exceeds 100 beats per minute.
Irrespective of the cause, maternal tachycardia in the postpartum woman should alert the clinician to the possibility of hypovolemic shock, as the potential for serious blood loss may occur when the maternal pulse exceeds 100 beats per minute.
When potentially significant blood loss is suspected, the etiology should be determined and steps implemented to facilitate prompt resuscitation and stabilization as needed. A frequent clinical error is delay in laboratory assessment of hemoglobin and/or hematocrit and clotting function, thus producing a delay in availability of blood products for transfusion. Early laboratory evaluation is recommended to establish a baseline and assess for the presence of anemia and/or coagulopathy with follow-up re-evaluation as clinically indicated by the degree of ongoing blood loss and change in vital signs.
Nonsurgical and Surgical Management of Postpartum Hemorrhage
There are no data from prospective randomized studies that define the optimum sequence of nonsurgical and surgical interventions to best treat PPH. Most recommendations are based on expert opinion, retrospective case studies, or studies with small samples of heterogeneous subjects. Therefore, the following interventions may not be in sequence for effective management of all patients with obstetric hemorrhage. Providers should individualize care based on patient history, current clinical condition, available resources, and updated clinical guidelines and recommendations for obstetric management of PPH.
Uterine Packing and Intrauterine Tamponade Balloons
Uterine packing for placental site bleeding and uterine atony has fallen out of favor in recent years but is still used by some practitioners with satisfactory results.19 Others have reported success in bleeding cessation with the use of intrauterine balloon tamponade modalities including condoms, Foley catheters, the Sengstaken-Blakemore tube (C.R. Bard, Inc., Covington, GA) and the Rusch urologic catheter. The SOS Bakri Tamponade Balloon Catheter (Cook Medical, Inc., Bloomington, IN) is a surgical obstetric silicone fluid-filled balloon (up to 500 mL capacity) approved by the U.S. Food and Drug Administration (FDA) in 2002 and designed for tamponade function.20 FDA labeling lists contraindications to its use in several settings, including coagulopathy, arterial bleeding requiring surgical exploration or angiographic embolization, bleeding requiring immediate hysterectomy, and others. A similar product, the BT-Cath (Utah Medical Products, Midvale, UT) balloon tamponade catheter (up to 500 mL capacity) is also commercially available. The FDA has also recently approved the Belfort-Dildy Obstetric Tamponade System (Glenveigh Surgical, LLC, Suwanee, GA) that employs dual balloons and is indicated for providing temporary control or reduction of postpartum uterine bleeding. Inflation of the vaginal balloon anchors the uterine balloon and provides tamponade if vaginal bleeding is present. All three tamponade balloons share the same contraindications.
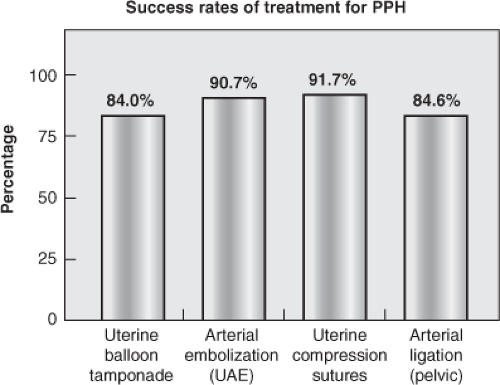
Balloon tamponade devices are particularly appealing to address placental-site bleeding including that from a low-lying placenta or placenta previa. In a systematic review of PPH management with balloon catheters for uterine tamponade, Doumouchtsis and colleagues reported an overall success rate of 84 percent (Fig. 15-2).21 The study evaluated the performance of the Foley urinary, Sengstaken–Blakemore esophageal balloon, Rusch balloon, and other unidentified catheters, and condoms. The high rate of hemorrhage abatement with the use of balloon catheters may encourage increased utilization of such devices in the control of uterine bleeding when future fertility is desired and/or as a temporizing intervention prior to surgical intervention in the unstable coagulopathic patient. There are currently no studies that compare and/or measure the effectiveness among individual types of balloon tamponade catheters.
Interventional Radiology
Selective arterial embolization (SAE) is a procedure performed by interventional radiologists in which a catheter is guided fluoroscopically through a peripheral artery (usually femoral) to the appropriate uterine vessel, which is then injected and embolized with various materials to occlude the artery. Uterine artery embolization (UAE) via arterial catheterization under fluoroscopic guidance has been used successfully in gynecologic patients in the treatment of pain and heavy
bleeding from uterine fibroids. It has also been used in the management of PPH to decrease blood supply to the uterus and thereby decrease blood loss. UAE for PPH has a reported 90.7 percent success rate in controlling bleeding and preventing hysterectomy, and appears particularly useful in cases of expanding retroperitoneal hematomas, where open surgical exploration may be difficult and dangerous.22 Its use, however, is limited to the availability of interventional radiologic teams and may not be feasible in the massively bleeding hemodynamically unstable intra-operative patient.
bleeding from uterine fibroids. It has also been used in the management of PPH to decrease blood supply to the uterus and thereby decrease blood loss. UAE for PPH has a reported 90.7 percent success rate in controlling bleeding and preventing hysterectomy, and appears particularly useful in cases of expanding retroperitoneal hematomas, where open surgical exploration may be difficult and dangerous.22 Its use, however, is limited to the availability of interventional radiologic teams and may not be feasible in the massively bleeding hemodynamically unstable intra-operative patient.
Uterine artery balloon placement is another interventional radiological procedure used in patients with extremely high probability for massive PPH, such as women with known placenta percreta. Prior to Cesarean delivery, intra-arterial sheaths (large bore catheters) are placed in both the right and left femoral arteries under fluoroscopic guidance. Balloon catheters (deflated) are then threaded through the sheaths and into the iliac arteries. The balloons are inflated after delivery of the infant to decrease blood supply to the uterus and thereby decrease blood loss. A relatively new procedure for the treatment of PPH, it has been used for temporary obstruction of blood supply in the iliac arteries. Post-procedure balloon re-inflation is performed in accordance with prescribed amounts of fluid in each balloon. Protocols and care guidelines for balloon placement, inflation volume, timed intervals of deflation and ongoing vascular assessments are defined by clinical guidelines and are beyond the scope of this chapter.
When nonsurgical management options do not stop the hemorrhage, providers should prepare for surgical intervention. Surgical procedures commonly used to treat obstetric patients with massive PPH are presented in Box 15-1.
Modalities include laceration repair, curettage, and hypogastric or uterine artery ligation or the use of a compression suture. The latter is particularly useful for women who have experienced uterine atony and who have responded well to bimanual compression. The B-Lynch compression suture is one example, first utilized successfully in 1997 as an innovative technique to manage uterine atony. The procedure involves placement of a continuous suture to envelope and mechanically compress the uterus in an attempt to avoid hysterectomy.
Box 15-1. Surgical Management of Postpartum Hemorrhage
Uterine curettage
Vaginal/cervical laceration repair
Uterine packing or balloon tamponade
Hypogastric artery ligation
Uterine artery ligation
B-Lynch stitch or other compression stitch
Hysterectomy
Pelvic packing
Data from References 66 and 69.
Hysterectomy
The incidence of obstetric hysterectomy ranges between 0.33 and 0.70 per 1000 deliveries.23,24,25,26 Maternal mortality associated with obstetric hysterectomy is substantial (0.6 to 4.5 percent) for a number of reasons, including the moribund condition of the patient at the onset of the operation, and the technical difficulty of the procedure, especially in the setting of ongoing hemorrhage.24,26,27 The average estimated blood loss during emergency obstetric hysterectomy has been reported at approximately 3500 mL, so one can expect the average patient undergoing emergency obstetric hysterectomy to lose at least half of her total blood volume.8 Concomitant coagulopathy often exists, particularly in patients requiring hysterectomy for abnormal placentation. Complications related to the procedure itself may include vascular, ureteral, bowel, and bladder injuries.
Coagulopathy and Obstetric Hemorrhage
Numerous professional organizations have published guidelines to direct contemporary blood replacement therapy (Table 15-6). Most of these guidelines strongly recommend intermittent laboratory analysis of bleeding profiles to guide transfusion therapy. Results of bleeding profiles may require a minimum of 30 to 40 minutes for analysis and thus may not reflect real-time clotting factor concentration during acute loss of blood. The expansion of point-of-care (POC) testing for hemostasis and fibrinolysis into operating rooms, intensive care units, outpatient clinics, etc., offers expedited results when compared to traditional laboratory testing. POC testing may offer obstetric providers similar benefits during obstetric hemorrhage; however, there are no randomized trials that have evaluated the impact of POC testing on patient outcomes in the setting of obstetric hemorrhage.
It is critical to note that the perfect test for hemostasis does not yet exist. Current laboratory tests do not accurately measure or reflect true in vivo coagulation. The prothrombin time (PT), activated partial thromboplastin time (aPTT), and International Normalized Ratio (INR) were developed to assess and adjust dosing of anticoagulant medications such as warfarin (INR) and heparin (aPTT). Laboratory tests have also been used in unsuccessful attempts to predict the risk for intra-operative and postoperative blood loss from coagulation deficiencies in patients undergoing invasive procedures. It is evident that more specific tests are needed to assess the ability of blood to remain liquid and yet
produce appropriate clotting when demanded by the body. Better understanding of the role of platelets in hemostasis, including the number and characteristics of platelets necessary to prevent active bleeding in the obstetric patient, is also important.
produce appropriate clotting when demanded by the body. Better understanding of the role of platelets in hemostasis, including the number and characteristics of platelets necessary to prevent active bleeding in the obstetric patient, is also important.
Table 15.6 National and International Guidelines in Blood Transfusion Therapy | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The dynamics of coagulopathy secondary to obstetric hemorrhage has recently been compared to the diathesis of bleeding found in trauma patients with significant penetrating and non-penetrating injuries.34 The rapid onset of coagulopathy that occurs at or around the time the uterus is emptied and the tissue factor–rich placenta detaches from the uterine wall to allow contraction of the myometrium; or that occurs when the placenta fails to detach, most likely has a multi-factorial cause. In these circumstances, bleeding in the obstetric patient appears similar in its rate of onset and subsequent volume of blood loss seen in young, previously healthy patients with traumatic injuries. Therefore, select management principles that have improved survival outcomes in trauma victims are recommended for incorporation into obstetric practice.35
Current trauma resuscitation protocols/guidelines are based on recent findings that a percentage of trauma patients present to emergency departments (EDs) or trauma centers with the presence of coagulopathy on admission.36,37,




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree