Although the timing, sequence, and pace of pubertal maturation vary among individuals, the sentinel events of puberty generally follow a predictable pattern. Adrenarche describes the activation of adrenal androgen secretion that begins before puberty and ultimately stimulates pubarche, the appearance of pubic hair. Gonadarche describes the activation of the hypothalamic-pituitary-gonadal axis, which facilitates the pubertal growth spurt, stimulates thelarche, the appearance of breast tissue, and finally menarche, the onset of menses.
Adrenarche
Adrenarche is the term used to describe the increase in adrenal androgen production that begins at approximately 6 years of age in both boys and girls.
75,76 Although adrenarche is independent of the maturation of the hypothalamic-pituitary-gonadal axis, the two often are temporally related.77 The increase in adrenal androgen production results from a change in the adrenal response to adrenocorticotropic hormone (ACTH) stimulation, characterized by a shift towards increased production of Δ
5-3β-hydroxysteroid intermediates (17α-hydroxpregnenolone; dehydroepiandrostendione, DHEA) and decreased production
of Δ
4-ketosteroids (17a-hydroxprogesterone, 17-OHP; androstendione), with no change in cortisol secretion.
78 Consequently, an increase in serum DHEA-sulfate (DHEA-S) levels heralds the onset of adrenarche.
Generally, the best indicator of adrenarche is a serum DHEA-S concentration greater than 40 μg/dL, which is higher than that normally seen in children 1-5 years of age (5-35 μg/dL).Adrenal androgens derive from the zona reticularis, the innermost layer of the adrenal cortex,
79 which begins to form at approximately 3 years of age and becomes well defined coincident with the increase in DHEA-S production at adrenarche. The molecular mechanisms that govern adrenal cortical differentiation involve a variety of different genes, but little is known about their transcriptional regulation.
80 The zona reticularis exhibits a unique enzymatic profile. The activity of 3β-hydroxysteroid dehydrogenase/Δ
5-Δ
4 isomerase (3β-HSD), which catalyzes the oxidation and isomerization of Δ
5-3β-hydroxysteroid precursors into Δ
4-ketosteroids, is low.
81 In contrast, the activities of P450c17, including both 17a-hydroxylase (catalyzing the conversion of pregnenolone to 17a-hydroxpregnenolone) and 17,20 lyase (catalyzing the conversion of 17a-hydroxpregnenolone to DHEA), are high, as is steroid sulfotransferase activity.
82 Cytochrome b5, which facilitates 17,20 lyase activity, also is preferentially expressed.
83 Taken together, the enzyme profile of the zona reticularis favors the formation of DHEA and DHEA-S.
The primary stimulus for adrenarche is unknown. Although ACTH is an obvious candidate, circulating levels of adrenal androgens change without any corresponding changes in ACTH or cortisol during fetal life, puberty, and with aging. In other conditions such as chronic disease, surgical stress, recovery from secondary adrenal insufficiency, and anorexia nervosa, changes in ACTH-induced cortisol secretion are not accompanied by any change in serum adrenal androgen concentrations.
84 Although derivatives of proopiomelanocortin (POMC, produced by pituitary corticotropes)
85 and other pituitary-dependent factors have been implicated,
86 conclusive evidence for an adrenal androgen stimulating hormone is lacking.
87,88 Whatever the stimulus might be, it might act to spur the growth and differentiation of the zona reticularis, which may derive from cells originally contained within the “fetal zone” of the adrenal cortex having a unique enzymatic profile (described above). Alternatively, it might act by suppressing 3β-HSD,
81 or by stimulating the 17,20 lyase activity of P450c17.
82 Interleukin-6 has been implicated as a mediator because it is highly expressed in the zona reticularis and can stimulate DHEA secretion.
89 Leptin also has been implicated, because adrenarche coincides with the preadolescent increase in body fat
90 and leptin levels
91 and leptin stimulates 17,20 lyase activity.
92 However, the temporal linkage between adrenarche and increasing body fat also might result from a compensatory hyperinsulinemia or from activation of the growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis.
93
The steady increase in adrenal androgen secretion after adrenarche ultimately stimulates
pubarche, the appearance of pubic hair, and also the development and activity of the pilosebacious unit, consisting of a hair follicle and sebaceous gland.
94 Adrenal androgen levels correlate with changes in bone density, suggesting they also may contribute to growth in cortical bone.
95 If adrenarche has any more fundamental role in the onset of puberty, the mechanism is unknown.
Adrenarche generally precedes activation of the hypothalamic-pituitary-gonadal axis, or gonadarche, by approximately 2-3 years. The temporal relationship suggests that adrenal androgen secretion might stimulate the pubertal transition, but several lines of evidence indicate otherwise. First, premature adrenarche generally is not associated with an earlier onset of thelarche or menarche. Second, adrenarche occurs in those with congenital hypergonadotropic hypogonadism (e.g., gonadal dysgenesis) or hypogonadotropic hypogonadism (e.g., Kallmann syndrome). Third, gonadarche occurs in children with Addison disease (hypoadrenalism) treated with glucocorticoids. Finally, in children under age 6 with true precocious puberty, gonadarche precedes adrenarche.
Growth
A substantial proportion of adult height, about 17-18%, is gained during puberty.96 Growth of the limbs precedes that of the trunk, beginning in the distal portions and later also involving the proximal part of the limbs; growth of the trunk occurs primarily during later puberty.
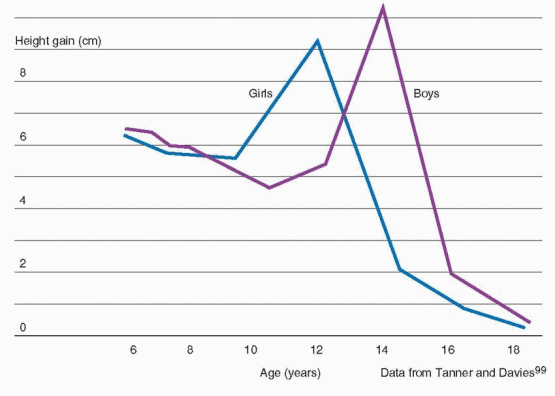
97 The pubertal growth spurt occurs approximately 2 years earlier in girls than in boys and, in general, peak height velocity is reached approximately 6 months before menarche.98
The difference in adult height of men and women relates to the onset and time of the growth spurt in boys and girls. Boys are approximately 2 cm taller than girls when girls reach their peak height velocity, grow 3-4 cm/year for another 1 to 2 years before entering puberty, and reach a greater peak height velocity (10.3 cm/year) than girls (9.0 cm/year).
99Bone mass accumulation during puberty is critical to the development of peak bone mass, which is a major determinant of the risk for developing osteoporosis in later life. Although genetics may be the most important determinant of peak bone mass, other factors such as nutrition and hormone exposure during puberty also contribute. About one-half of total body calcium is accrued during puberty in females, and one-half to two-thirds in males.
100,101 In girls, the peak velocity in bone mineral accretion occurs at menarche, approximately 9-12 months after peak height velocity is attained.102,103 The pubertal increase in bone density is greater in black females than in white females.
104 Taken together, these data suggest that the window of opportunity to maximize peak bone mass is relatively narrow.
101
Weight changes during pubertal maturation reflect changes in body composition and the relative proportions of lean body mass and fat. Skinfold thickness decreases in early puberty and increases after peak height velocity, particularly in girls. Adolescent girls have more body fat than boys, most being deposited in the upper arms, thighs and back; the gender difference increases throughout puberty. The increase in BMI before 16 years of age relates primarily to changes in fat-free mass and thereafter to an increase in fat mass.
105 If desired, BMI can be tracked from adolescence to adulthood using published tables for comparison by age and ethnicity.
106
Growth Hormone
Growth hormone, produced by somatotropes, is the pituitary hormone produced in greatest abundance. The GH gene family includes five distinct genes, all of which are located on chromosome 17 (17q22).
107 The pituitary GH gene
(GH1) encodes two alternatively spliced mRNAs, yielding a predominant 22 kDa GH molecule and another 20 kDa molecule that accounts for approximately 10% of circulating GH. Placental syncytiotrophblasts express a GH variant in addition to three other genes encoding human chorionic somatotropin, also known as human placental lactogen (discussed in
Chapter 8). The regulation of pituitary GH secretion is highly complex. GH secretion is controlled primarily by hypothalamic GHreleasing hormone (GHRH) and by peripheral factors acting on somatotropes that stimulate (e.g., ghrelin),
108 or inhibit (e.g., somatostatin)
109 GH release.
110,111 and
112 Most of the peripheral actions of GH are mediated by insulin-like growth factor I (IGF-I), which inhibits GH release. Nutritional factors also play a role in the regulation of GH secretion; whereas fasting
113 and high protein meals
114 stimulate GH release, hyperglycemia and leptin inhibit GH secretion.
115 Estrogens stimulate, and excess glucocorticoids inhibit GH release.
GH secretion peaks during puberty and declines with age, by approximately 50% every 7 years.113Like the gonadotropins, GH is secreted in a pulsatile fashion, and at the onset of puberty, GH pulse amplitude increases, especially during sleep.
116 Consequently, GH concentrations rise progressively.
The rate of the increase in circulating GH levels is the most important determinant of the pubertal growth rate; slower growing children exhibit fewer and lower amplitude GH pulses and a more gradual increase in serum GH concentrations.117GH acts via binding to a specific receptor to stimulate hepatic synthesis and secretion of IGF-I, which promotes both growth and differentiation.
107 GH receptor mutations result in GH insensitivity and growth failure (Laron dwarfism);
118 in affected individuals, serum GH concentrations are elevated and levels of IGF-I are low. GH stimulates growth via direct and indirect (via IGF-I) actions on the epiphyseal plates of long bones. GH also has a number of metabolic actions, which include increased lipolysis, stimulation of protein synthesis, insulin antagonism, and water and sodium retention.
Insulin-like Growth Factor I
IGF-I is synthesized and secreted by the liver in response to GH stimulation and circulates in serum bound to high affinity IGF binding proteins (IGFBPs). The genes encoding IGF-I, IGF-II, and insulin all belong to the same family. The
IGF1 gene has several components and yields several different mRNAs, including the 6 kb form that is regulated by GH. IGF-I acts via its own receptor, which is widely distributed in a variety of tissues and organs.
119 IGF-I receptor concentrations are controlled by GH, thyroxine, and other growth factors such as fibroblast growth factor and platelet-derived growth factor. IGF-I acts via a complex signaling cascade to stimulate cell growth and to inhibit apoptosis.
The family of IGFBPs includes six proteins having greater affinities for IGF-I than the IGF-I receptor. IGFBPs are present in all extracellular fluids and serve both to transport IGF-I and to control the amount of IGF-I available to bind to the IGF-I receptor. IGFBP-3 is the most abundant in serum and has the highest affinity for IGF-I, but generally is saturated. Although present in lower concentrations, IGFBP-1 is unsaturated and therefore has greater impact on the levels of free IGF-I. The serum IGFBP-1 concentration is regulated by insulin, increasing during fasting when insulin levels are low, and decreasing after feeding or administration of insulin.
120 IGF-I levels are decreased in diseases associated with malnutrition such as inflammatory bowel disease and in hypothyroidism. IGF-I augments the effects of FSH and LH in the ovary, the effect of ACTH on adrenal steroidogenesis, and
the thyroid response to thyroid-stimulating hormone (TSH).
IGF-I levels rise 7-fold from very low concentrations at birth to peak values at puberty, fall rapidly by approximately 50% by age 20, then decline slowly with advancing age.121
Gonadal Steroids
The pubertal growth spurt is stimulated primarily by rising levels of GH and IGF-I, but a substantial body of evidence indicates that sex steroids also play an important role. In children with central (gonadotropin-dependent) precocious puberty treated with a longacting GnRH agonist, mean height velocity and nocturnal serum GH and IGF-I levels, initially above the means for chronological age, decrease significantly after 6-12 months and remain suppressed for the duration of treatment.
122,123 A study in children with central precocious puberty and GH deficiency (due to an intracranial lesion) observed that bone age was advanced in GH-deficient subjects, but not as much as in control subjects with precocious puberty and normal GH secretion; IGF-I levels were lower in GH-deficient subjects, but greater than in age-matched prepubertal GH-deficient children.
124 In a subset of GH-deficient subjects, treatment with a GnRH analog suppressed gonadal sex steroid levels and decreased height velocity, with no appreciable change in GH or IGF-I levels. In girls with Turner syndrome, treatment with exogenous estrogen increases growth velocity and bone age, compared to those observed in placebo-treated controls.
125,126 Taken together, these observations indicate that the pubertal growth spurt is mediated, at least in part, by a sex steroid-induced increase in GH secretion. Moreover, they demonstrate that precocious puberty can induce a substantial growth spurt, even in the absence of a normal pubertal increase in circulating GH or IGF-I. However, normal pubertal growth requires the combined actions of sex steroids and GH. Ultimately, sex steroids limit adult height by stimulating epiphyseal fusion.
The Timing of Puberty
What triggers the onset of puberty remains one of the most compelling unanswered questions in reproductive endocrinology. The age at onset of puberty and menarche is influenced by genetics, overall health, social environment, and environmental exposures.
An analysis of two genome-wide association studies including more than 17,000 women from the Nurses’ Health Study and the Women’s Genome Health Study identified 10 common variants or single nucleotide polymorphisms (SNPs) clustered in the regions of chromosomes 6q21 and 9q31.2 that were associated with age at menarche.
127,128 Genetic variation in or near the locus (6q21) of the
LIN28B gene (encoding a developmentally regulated RNA binding protein)
129 has been associated with age at menarche in a number of human populations.
128,130,131 Some genetic variants associated with adult height also have been associated with age at menarche, suggesting the association between height and age at menarche has a genetic basis.
128,130 Other genes associated with age at menarche include
FTO (fat mass and obesity associated gene) and
NEGRI (neuronal growth regulator 1), both of which also are associated with childhood obesity.
130 Children with a family history of early puberty are more likely to experience an early puberty themselves; age at menarche correlates relatively well between mothers and daughters and between sisters.
132Children who live closer to the equator, at lower altitudes, in urban areas, and mildly obese children generally begin puberty earlier than those who live in northern latitudes, at higher elevations, in rural areas, and those of normal weight. Accumulating evidence suggests that
certain environmental toxicants acting as “endocrine disruptors” also may influence the timing of sexual development.
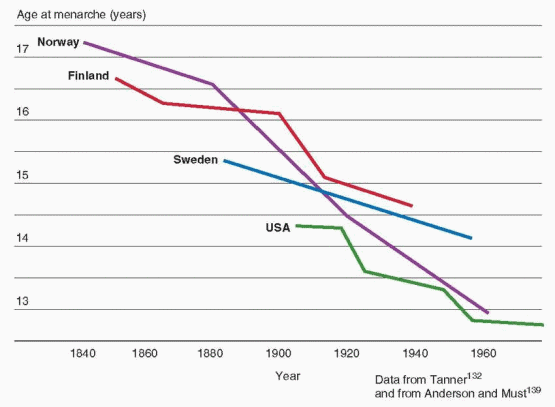
133The age at onset of puberty has been declining gradually in the general population of the United States over the past century. Although the rate of decrease has slowed considerably more recently, the trend has continued. Overall, the average age at menarche for American girls decreased from approximately 12.75 years in the 1960s to approximately 12.5 years in the early 1990s.
134,135 A 1997 study conducted by the Pediatric Research in Office Settings (PROS) network examined the timing of pubertal development in more than 17,000 American girls (90% white, 10% black) and found that the earliest signs of puberty were occurring at ages significantly younger than in the past, with striking racial differences, as follows:
136
Pubertal Milestone |
Black American Girls |
White American Girls |
Thelarche |
|
|
|
Mean age
Age 6
Age 7
Age 8
Age 9
Age 10
Age 11
Age 12 |
8.9 yr
6.4 %
15.4%
37.8%
62.6%
80.2 %
96.0 %
98.9 % |
10.0 yr
2.9 %
5.0 %
10.5 %
32.1 %
61.5 %
85.4 %
96.0 % |
Pubarche |
|
|
|
Mean age
Age 6
Age 7
Age 8
Age 9
Age 10
Age 11
Age 12 |
8.8 yr
9.5 %
17.7 %
34.3 %
62.6 %
85.6 %
95.2 %
98.9 % |
10.5 yr
1.4 %
2.8 %
7.7 %
20.0 %
46.4 %
74.3 %
92.2 % |
Thelarche and/or Pubarche |
|
|
|
Age 6
Age 7
Age 8
Age 9
Age 10
Age 11
Age 12 |
14.3%
27.2 %
48.3 %
77.4 %
94.6 %
98.4 %
100.0 % |
3.7 %
6.7 %
14.7 %
38.2 %
67.9 %
88.0 %
96.6 % |
Menarche |
|
|
|
Mean age
Age 9
Age 10
Age 11
Age 12 |
12.2 yr
2.7 %
6.3 %
27.9 %
62.1 % |
12.9 yr
0.2 %
1.8 %
13.4 %
35.2 % |
These data indicate that a substantial proportion of American girls begin pubertal development 6-12 months earlier than previously observed. On average, black American girls begin puberty between ages 8 and 9, and white American girls by age 10. However, thelarche and/or pubarche can occur normally in black girls as early as age 6 and in white girls as early as age 7.
Subsequent studies analyzing data from the National Health and Nutrition Examination Survey (NHANES) have observed a 2.3 month decrease in the average age of menarche between surveys for the years 1988-1994 (12.53 years) and 1999-2002 (12.34 years), and an overall 4.9 month decrease since 1960.
134,137,138 and
139 The decrease in age of menarche has been observed in all ethnic groups, declining from 12.57 to 12.52 years in non-Hispanic white girls, from 12.09 to 12.06 years for non-Hispanic black girls, and from 12.24 to 12.09 for Hispanic American girls.
139 Changes in the population distribution of race and ethnicity over time explain the larger change in overall average age compared to those within groups.
 |
Historically, the trend to an earlier onset of sexual development has been attributed to improved nutrition and less stressful living conditions.
140 The age at menarche has declined as the prevalence of obesity has increased, suggesting that a critical body weight
58 or body composition
59 is an important factor in determining the onset and progression of puberty.
60 Indeed, higher weight and body fat mass are associated with an increased likelihood of early menarche.60,134,141,142 and 143 Data from the U.S. NHANES survey indicated that a girl with a BMI at the 85th percentile is more than twice as likely to have reached menarche as a girl of the same age and race/ethnicity having a BMI at the 50th percentile.
139 However, girls reach menarche over a wide range of weight and BMI, and age at menarche cannot be predicted reliably for individuals on that basis.
144 Importantly, early pubertal development is associated with slightly decreased adult height and an increased risk for obesity, compared to a late menarche.145,146
Stages of Pubertal Development
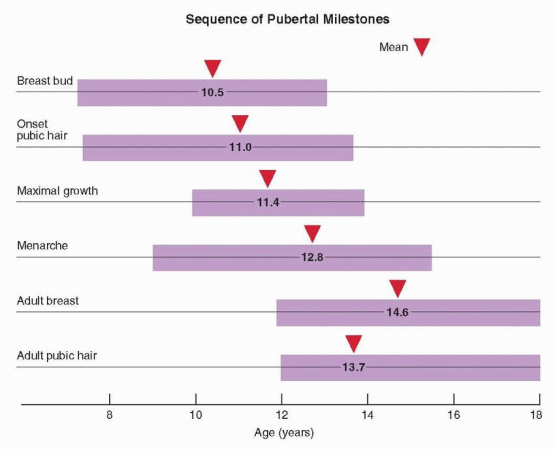
Puberty includes a series of predictable events that vary in timing, sequence, and pace. In general, the first sign of puberty in most adolescent girls is an acceleration of growth, followed by breast budding (thelarche), the appearance of pubic hair (pubarche), and finally, the onset of menses (menarche).
The staging systems used most frequently to describe the physical changes of puberty were first described by Marshall and Tanner in 1969 (girls)
148 and 1970 (boys).
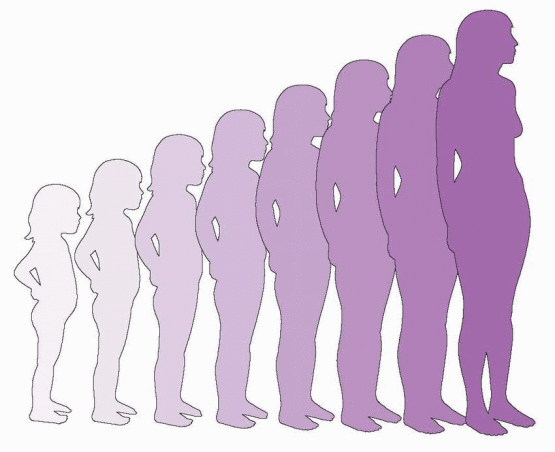
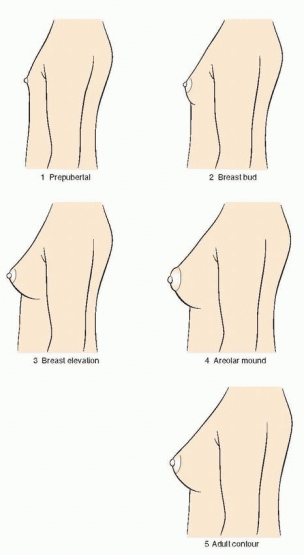
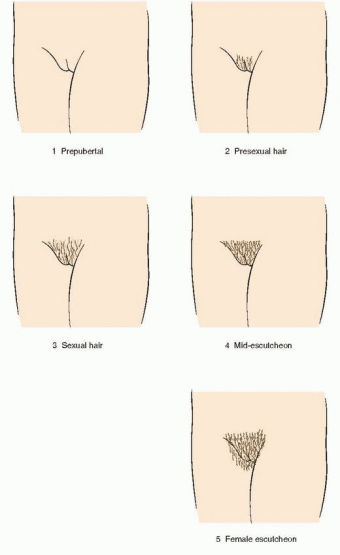
149 The Tanner stages describe secondary sexual characteristics, including breast development in girls, pubic hair growth in both sexes, and genital development in boys. As shown in the diagrams, there are five Tanner stages of breast and pubic hair development in girls, with stage 1 representing the prepubertal state and stage 5 representing adult development.
Breast development follows a recognized sequence of events. Breast budding (Tanner stage 2) is distinguished by enlargement and by widening of the areolae. The breast then enlarges, becoming elevated beyond the areolae (Tanner stage 3). The breast enlarges further and the areolae and nipple form secondary mounds (Tanner stage 4), just before the breast achieves an adult contour (Tanner stage 5).
In the majority of adolescents, pubarche closely follows thelarche, but in a substantial minority the sequence is reversed and pubarche precedes thelarche. In either case, the two are closely linked and progress in parallel. Pubarche (Tanner stage 2) is distinguished by the emergence of a small amount of long, relatively straight hair on the labia majora. The hair then becomes curly, coarser, and extends outward (Tanner stage 3). Hair extends further to cover the labia (Tanner stage 4) before assuming an adult pattern with extension onto the medial thigh (Tanner stage 5).
Menarche occurs an average of 2.6 years after the onset of puberty and after the peak of growth has passed.98,102,148 On average, the pubertal sequence of accelerated growth, thelarche, pubarche, and menarche requires a period of 4.5 years (range, 1-6 years). The relationship between menarche and the growth spurt is relatively fixed. After menarche, growth slows and generally does not increase more than about 6 cm (2.4 inches). The menses immediately following menarche usually are anovulatory, irregular, and occasionally heavy. Anovulatory cycles frequently persist for as much as 12-18 months and are not
uncommon even up to 4 years after menarche.
150,151 However, the frequency of menses generally increases rapidly over the first year after menarche; 65% of adolescent girls report having 10 or more periods per year at the end of the first postmenarcheal year, and 90% after 3 years.
30 The hallmark of the maturation of the hypothalamic-pituitary-ovarian axis and of the completion of puberty is the development of estrogen positive feedback, which stimulates the midcycle LH surge and ovulation. In general, ovulatory cycles become progressively more frequent. The time required to establish ovulatory cycles relates to the age at menarche; when menarche occurs after the age of 13, only one-half have ovulatory cycles within 4.5 years.
152
 |
 |
 |