Recurrent Early Pregnancy Loss 0030
 |
Spontaneous abortion or miscarriage is defined as the involuntary termination of pregnancy before 20 weeks of gestation (dated from the last menstrual period) or below a fetal weight of 500 g. Losses after 20 weeks are considered stillbirths or premature births and generally have different causes than losses that occur earlier in gestation.
Historically, recurrent pregnancy loss or “habitual abortion” was defined as three or more consecutive spontaneous miscarriages. Popular theory during the 1930s and 1940s held that risk for spontaneous miscarriage increased progressively with each successive loss. Calculations based on that assumption by Malpas and later by Eastman suggested that three consecutive miscarriages demonstrated a predisposition to pregnancy loss that raised the risk for spontaneous miscarriage in the next pregnancy to as high as 73-84%.1,2 In that era, the “control” for numerous studies evaluating the effectiveness of various treatments for recurrent pregnancy loss (hormones, vitamins, psychotherapy) was theoretical rather than real; the observed incidence of miscarriage in treated women was compared to the predicted or expected incidence rather than to the actual incidence observed in untreated or placebo-treated women. Unfortunately, one of the consequences of such flawed study design was the erroneous conclusion that treatments, including diethylstilbestrol (DES), were effective when in fact they were not. Years later, clinical studies based on empiric observations demonstrated that the risk of miscarriage after three previous losses is actually much lower than predicted (30-45%) and varies with the number of previous live births (none, 40-45%; one or more, about 30%).3,4,5 and 6
There is no specific number of miscarriages or firmly established criterion that justifies evaluation for recurrent pregnancy loss or defines the scope of investigation. Decisions must be individualized and consider the female partner’s age, the timing and circumstances surrounding earlier pregnancy losses, elements of the personal and family medical history, and the couple’s level of anxiety. Today, recurrent pregnancy loss is usually defined as three or more pregnancy losses (not necessarily consecutive).7 Most also consider clinical investigation and treatment appropriate in couples with two consecutive spontaneous miscarriages, preferably documented by ultrasound or histopathological examination. Evaluation is especially indicated when any of the following are also present:
Embryonic heart activity observed before any earlier pregnancy loss.
Normal karyotype on products of conception from an earlier loss.
Female partner age over 35 years.
Infertility.
|
The vast majority of all early pregnancy losses result from chromosomal abnormalities arising in the egg, the sperm, or during early embryonic development and are random events. Even repeated miscarriages can occur by chance alone, but at least some affected couples have a predisposing factor. Among all the factors that have been implicated, the only undisputed causes of recurrent pregnancy loss are genetic (balanced chromosomal translocation in either partner, maternal age-related increase in prevalence of aneuploid oocytes), anatomic (congenital and acquired uterine abnormalities), or immunologic (the thrombotic complications of antiphospholipid syndrome). Alloimmunopathology, inherited thrombophilias (Factor V Leiden and others), endocrinopathies (thyroid disorders, diabetes, luteal phase deficiency), infections (genital mycoplasmas), and environmental exposures (smoking, heavy alcohol or caffeine consumption) have been implicated but are not established causes of recurrent pregnancy loss. Even after a comprehensive evaluation, recurrent pregnancy loss remains unexplained in well more than half of affected couples.
For all couples who have suffered recurrent pregnancy loss, education can provide important perspective; most couples welcome the offer of evaluation to identify any predisposing factor. When a likely cause can be defined, specific counseling and treatment can improve the prognosis for a successful pregnancy. When no specific cause can be found, reassurance and encouragement are no less valuable.
The Epidemiology of Pregnancy Loss
Early pregnancy loss is a very common event, even more so than most couples realize. Almost all chromosomally abnormal conceptions spontaneously abort, most before 10 weeks’ gestation, and over 90% of conceptions having a normal karyotype continue.8,9 Miscarriage may thus be viewed as a natural selection process for quality control. Learning that miscarriage is common, normal, and inevitable in most cases does not heal the emotional wounds left by earlier losses or eliminate the anxiety that affected couples have when contemplating another attempt at pregnancy,10,11 but an accurate perspective is nonetheless important and often very helpful.
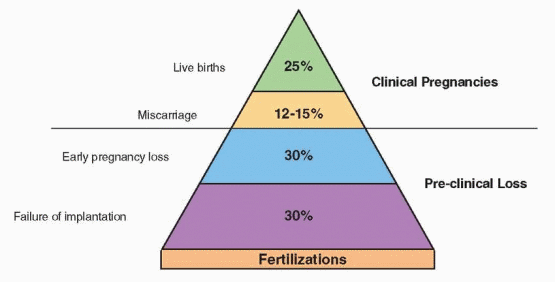
Overall, approximately 12-15% of clinically recognized pregnancies end in spontaneous miscarriage between 4 and 20 weeks of gestation. However, the true early pregnancy loss
rate, including both clinically recognized and unrecognized occult early miscarriages, is two to four times greater, depending on age. Careful studies in normally cycling healthy young women attempting pregnancy have shown that human chorionic gonadotropin (hCG) can often be detected transiently in the urine of women who are otherwise quite unaware that they had conceived and miscarried.12,13 and 14 No less than 30% and as much as 60% of all conceptions abort within the first 12 weeks of gestation, and at least half of all losses go unnoticed. The reproductive loss that occurs even before a first missed menses is substantial.15 Most recognized pregnancy losses occur before 8 weeks’ gestation, and relatively few occur after 12 weeks.16
rate, including both clinically recognized and unrecognized occult early miscarriages, is two to four times greater, depending on age. Careful studies in normally cycling healthy young women attempting pregnancy have shown that human chorionic gonadotropin (hCG) can often be detected transiently in the urine of women who are otherwise quite unaware that they had conceived and miscarried.12,13 and 14 No less than 30% and as much as 60% of all conceptions abort within the first 12 weeks of gestation, and at least half of all losses go unnoticed. The reproductive loss that occurs even before a first missed menses is substantial.15 Most recognized pregnancy losses occur before 8 weeks’ gestation, and relatively few occur after 12 weeks.16
 |
Numerous studies have documented that the risk of spontaneous miscarriage varies with past obstetrical history.3,16,17 and 18 In general, women in their first pregnancy, those whose only other pregnancy was electively terminated, and women whose only or last pregnancy was successful have a relatively low risk of spontaneous miscarriage (4-6%). Conversely, women whose only or last pregnancy ended in loss have a higher risk of miscarriage in their next pregnancy (19-24%).16 Unless there has been a subsequent successful pregnancy, even a single loss increases risk for another spontaneous miscarriage in the next pregnancy. Taken together, available evidence also suggests that miscarriage risk increases with the number of pregnancy losses, but very gradually.4,5 and 6 Overall, the risk is still less than 40% after four previous losses and no higher than about 50% even with six or more; risk may be modestly higher for women with recurrent pregnancy loss and no previous live-born children.
Independent of past obstetrical history, the risk of clinically recognized spontaneous miscarriage increases with age. Risk is relatively low before age 30 (7-15%) and only slightly higher for women aged 30-34 (8-21%), but then rises more sharply for ages 35-39 (17-28%) and women age 40 and older (34-52%).19,20,21,22 and 23 Among women with a past history of pregnancy losses, advancing age adds to the risk related to previous losses; the miscarriage risk for women over age 40 (52%) is more than twice that for women under age 30 (25%).6 If both recognized and occult pregnancy losses are considered, total pregnancy wastage in women over age 40 may reach or exceed 75%.13,23,24
In summary, approximately 12-15% of all clinically recognized pregnancies end in miscarriage, but the true incidence of miscarriage, including unrecognized early pregnancy losses, is two to four times higher (30-60%). Miscarriage risk increases with the number of previous pregnancy losses but rarely exceeds 40-50%. Risk for pregnancy loss also rises with increasing maternal age, moderately after age 35 and more rapidly after age 40.
Prognostic Value of Transvaginal Ultrasound Observations
Careful serial observations during early pregnancy have indicated that the risk for miscarriage decreases as the duration of pregnancy increases. The risk of pregnancy loss falls progressively after observation of a gestational sac (12%), a yolk sac (8%), and as embryonic crown-rump length increases (greater than 5 mm, 7%; 6-10 mm, 3%; greater than 10 mm, less than1%).25 The observation of embryonic heart activity (by approximately 6 weeks’ gestation) is another important developmental milestone and good prognostic indicator because most ill-fated pregnancies fail before then, but its predictive value varies with the past obstetrical history, clinical context, and age. In both normal and infertile asymptomatic young women, the timely appearance of embryonic heart activity decreases the risk of pregnancy loss from the global risk of 12-15% to between 3% and 5%.26,27 In women with past histories of recurrent pregnancy loss, the miscarriage rate after detection of embryonic heart activity is still three to five times higher (15-25%).28,29 In women with threatened abortions, demonstrable embryonic heart activity is again a good prognostic indicator overall (15% loss rate), but the incidence of subsequent loss is higher when there are other abnormal sonographic findings (slow or late appearing heart activity, size/date discrepancies, subchorionic hematoma).30,31,32 and 33 Finally, the prognostic value of embryonic heart activity declines with increasing maternal age; whereas the risk for subsequent loss is low (less than 5%) in women age 35 and under, it is two to three times higher (approximately 10%) in women ages 36-39, and increased another 3-fold (29%) in women age 40 and over.34
Genetic Factors
Most spontaneous miscarriages result from chromosomal abnormalities in the embryo or fetus. Numerous studies in which large numbers of abortuses have been cultured and karyotyped have suggested that approximately 50% of all first trimester pregnancy losses, 30% of second trimester abortuses, and 3% of stillbirths are chromosomally abnormal.21,22,35,36 and 37 However, these studies have very likely underestimated the prevalence of chromosomal abnormalities among abortuses because the data are biased by unrecognized maternal cell contamination and because normal euploid cells (from the mother or abortus) are less likely to fail culture than abnormal cell lines.38,39 and 40 Analyses using newer techniques not dependent on cell culture (fluorescence in situ hybridization, FISH; comparative genomic hybridization, CGH), and more recent careful cytogenetic studies of early missed abortions suggest that the true incidence of chromosomal abnormalities in miscarried early pregnancies is closer to 75%.41,42
Over 90% of the chromosomal abnormalities observed among abortuses are numerical (aneuploidy, polyploidy); the remainder are split between structural abnormalities (translocations, inversions) and mosaicism.42,43 Overall, autosomal trisomies are the most common abnormality (usually involving chromosomes 13-16, 21, or 22), followed by monosomy X (45,X) and polyploidies.21,42,44,45 Among women with history of recurrent pregnancy loss, chromosomally normal (euploid) abortuses are more common, particularly in those age 35 and under.45,46,47 and 48 The distribution of chromosomal abnormalities observed among the abortuses of women with recurrent pregnancy loss is otherwise no different from that seen in the general population when stratified by maternal or gestational age.45 The high incidence of sporadic miscarriage and random chromosomal abnormalities means that some of the pregnancy losses in women with recurrent miscarriages result from chance. The likelihood of a euploid abortus increases with the number of previous miscarriages and after a previous abortion having a normal karyotype.24,47
Parental Chromosomal Abnormalities
The overwhelming majority of chromosomally abnormal conceptions result from the chance union of one normal and one aneuploid gamete or from nondisjunction during early embryonic development. However, in 4-8% of couples with recurrent pregnancy loss, one or the other partner harbors a chromosomal abnormality that markedly increases the probability of a chromosomally unbalanced conceptus.49,50,51,52,53 and 54 Balanced translocations (reciprocal, Robertsonian) are the most common abnormalities; sex chromosome mosaicism, chromosome inversions, and other structural abnormalities can also be observed.55,56
In a balanced reciprocal translocation, pieces of two different autosomes (one from each of two different pairs) are translocated (exchanged). In a balanced Robertsonian translocation, the centromeres of two acrocentric chromosomes (numbers 13,14,15, 21, 22) fuse to form a single chromosome consisting of the long arms of the two affected chromosomes; the short arms (containing little or no essential genetic material) are lost. In both cases, the translocation carrier is genetically balanced and phenotypically normal. Unfortunately, when their oogonia or spermatogonia undergo meiosis to yield haploid oocytes or sperm, a large proportion of the gametes end up genetically unbalanced and abnormal, having either a deficiency or an excess of genetic material. Depending on how the chromosomes segregate during meiosis, the gametes may be chromosomally normal (containing only the normal copy of each of the two affected chromosome pairs), abnormal but balanced (containing the translocated member of each of the two affected chromosome pairs), or abnormal and unbalanced (containing two copies or no copies of an affected chromosome or chromosome segment). When such chromosomally unbalanced gametes combine with a normal gamete from an unaffected partner, the conceptus will have a trisomy and/or a monosomy and will almost always abort; an unbalanced conceptus may occasionally survive, but those that do are at high risk for malformations and mental retardation.57
In theory, one-fourth of the gametes produced by reciprocal translocation carriers should be normal, one-fourth should be abnormal but balanced, and one-half should be abnormal and unbalanced, yielding a 50% probability of a normal pregnancy (normal or balanced conceptus) and a 50% probability of an abnormal pregnancy (abortion or a viable but anomalous fetus), assuming union with a chromosomally normal gamete from the unaffected partner. Similarly, given the three different ways in which a Robertsonian translocation chromosome and the normal members of the affected chromosome pairs may align and segregate during meiosis, one-sixth of the gametes produced by carriers should be normal, one-sixth should be abnormal but balanced, and the remaining two-thirds should be abnormal and unbalanced, yielding a 33% probability of a normal pregnancy (normal or balanced conceptus) and a 67% probability of an abnormal pregnancy (abortion or a viable but anomalous fetus), again assuming a union with a chromosomally normal gamete from the unaffected partner. However, when a Robertsonian translocation involves both members of a single pair of chromosomes, the carrier will produce no normal gametes because all will have either two copies or no copy of the affected chromosome.
Some reciprocal translocations are predisposed to specific rather than random segregation patterns and may yield a skewed distribution of normal, balanced, and unbalanced gametes.58,59 and 60 The probability of a successful pregnancy and the risk of a chromosomally abnormal but viable fetus vary with the specific chromosomes involved and the size and location of the translocated segments.43,57 Abnormalities of some chromosomes (chromosome 21) are better tolerated than others and risk of an unbalanced but viable conceptus is higher when the exchanged chromosomal segments are small. By their very nature, reciprocal translocations tend to be rather unique, so there is usually no easy way to accurately predict the probability of specific pregnancy outcomes for an individual affected couple. At best, the karyotype of the affected partner can allow one to predict the most likely segregation patterns for a specific translocation and to estimate the risk of unbalanced
offspring. When the male partner is the translocation carrier, the distribution of normal, balanced, and unbalanced sperm and the prognosis for successful conception can be more accurately defined.60 When the female partner is the carrier or the distribution of gametes is otherwise unknown, the couple’s own reproductive history (and that of any other similarly affected family member) is the best gauge. One exception is a specific recurring translocation involving chromosomes 11 and 22, t(11;22)(q23;q11), the most common reciprocal translocation in humans; over 100 unrelated affected families have been reported, and the reproductive performance of carriers has been well defined.61,62 and 63
offspring. When the male partner is the translocation carrier, the distribution of normal, balanced, and unbalanced sperm and the prognosis for successful conception can be more accurately defined.60 When the female partner is the carrier or the distribution of gametes is otherwise unknown, the couple’s own reproductive history (and that of any other similarly affected family member) is the best gauge. One exception is a specific recurring translocation involving chromosomes 11 and 22, t(11;22)(q23;q11), the most common reciprocal translocation in humans; over 100 unrelated affected families have been reported, and the reproductive performance of carriers has been well defined.61,62 and 63
Chromosomal inversions occur less frequently than translocations and may or may not have reproductive implications, depending on their size and location. Pericentric inversions (those that involve the centromere) often have no clinical consequences; a pericentric inversion of chromosome 9, inv(9)(p11q13), is so common (1-1.5% in the general population) that some consider it a normal variant with no importance.64,65 However, the crossovers and recombinations that can occur with paracentric inversions (those not located at the centromere) frequently result in an excess of genetic material resulting in abortion or an anomalous fetus.43
As might be anticipated, the most common reproductive history in translocation carrier couples includes both a normal child and early pregnancy losses (6-7%); other histories involving only spontaneous miscarriages or combinations of malformed children, stillbirths, and abortions are slightly less common (4-5%).52 The probability of identifying a balanced chromosomal translocation in a couple with three or more previous pregnancy losses is not significantly higher than in those having had only two. In some couples, family history (recurrent pregnancy loss, stillbirths, or birth defects) suggests the possibility of an occult chromosomal abnormality after only one spontaneous miscarriage. Couples with miscarriages interspersed with normal pregnancies and outcomes should be evaluated in the same fashion as couples with consecutive miscarriages.66
Balanced chromosomal translocations can be found in either partner, and both must be karyotyped to exclude the possibility.52 Any balanced translocation so identified may have arisen de novo or have been inherited from one of the carrier’s own parents. If the translocation was inherited, any of the carrier’s siblings and, in turn, their offspring might also be affected.67 Any pregnancy in an affected couple becomes a candidate for prenatal diagnostic studies, regardless of the mother’s age or previous reproductive history.66 Consequently, counseling of translocation carrier couples with recurrent pregnancy loss should consider karyotyping the carrier’s parents and, where appropriate, other potentially affected individuals in the kindred. For minor children who may be carriers, karyotyping is best postponed until they reach an age where they are able to grant informed consent.
It is entirely possible and even likely that some couples with recurrent pregnancy loss may harbor a genetic abnormality that predisposes to a higher risk of miscarriage but cannot be detected using standard cytogenetic techniques. Possibilities include isolated gonadal or germline mosaicism (including a trisomic cell line) and single gene defects.43
Aging and Gamete Aneuploidy
The mechanisms responsible for the age-related increase in the incidence of miscarriage and the use of ovarian reserve tests in the evaluation of reproductive age and prognosis are discussed in detail in Chapter 27. The genetic factors that contribute to the increase in pregnancy wastage associated with reproductive aging and the utility of ovarian reserve testing in women with recurrent pregnancy loss are only summarized briefly here.
Several lines of evidence suggest that age-related instability or degradation of the cellular mechanisms that govern meiotic spindle formation and function results in an increasing incidence of meiotic segregation errors and a rapid rise in the numbers of aneuploid oocytes during the later reproductive years.68,69,70,71,72,73 and 74 The best available estimates indicate that the prevalence of aneuploid oocytes is relatively low before age 35 (less than 10%) but increases abruptly thereafter, reaching 30% by age 40, 50% by age 43, and nearly 100% after age 45.68 These observations offer a logical explanation for the overall age-related increase in the incidence of miscarriage and the higher prevalence of aneuploidy among the abortuses of aging women.19,20,21 and 22 Indeed, most trisomies observed among abortuses can be traced to maternal meiotic errors and oocyte aneuploidy.75
Some women with otherwise unexplained recurrent pregnancy loss have a diminished ovarian reserve that may help to explain their poor reproductive performance.76,77 The prevalence of abnormal ovarian reserve tests in women with unexplained recurrent pregnancy loss is higher than in women with other defined causes of recurrent pregnancy loss76 and comparable to that observed in the general population of infertile women.77 These observations suggest that women at advanced stages of ovarian follicular depletion are at higher risk for miscarriage, regardless of their age. For them, the curve that describes the age-related rise in the incidence of spontaneous miscarriage is shifted left, and the sharp rise in miscarriage risk that normally begins at about age 37 starts earlier.19,20,21 and 22 Some women will suffer premature ovarian follicular depletion because they are born with a smaller than normal ovarian follicular pool and are genetically destined to be among the 10% of women who experience an early menopause.78,79,80,81 and 82 Women who have had a trisomic abortus reach menopause at an earlier average age.83 Other women may have their ovarian follicular pool depleted by disease that destroys ovarian tissue or requires its removal. Either way, the end result is the same—accelerated follicular depletion, declining fertility, and increasing risk for miscarriage begin at an earlier than normal age. Women with a demonstrated low ovarian reserve have an extremely high rate of pregnancy loss, regardless of age.84
Besides offering information that may help to explain recurrent pregnancy loss, ovarian reserve testing may identify young women at increased risk for fetal aneuploidy in subsequent pregnancies who would otherwise not be considered candidates for prenatal diagnostic studies.85,86,87,88 and 89 The incidence of Down syndrome is increased in women with elevated serum levels of follicle-stimulating hormone (FSH), regardless of age and regardless of whether the low ovarian reserve the high FSH reveals came about naturally or resulted from ovarian surgery.85,87,88 and 89
The prevalence of skewed X chromosome inactivation, defined as preferential inactivation (more than 90%) of one of the two X chromosomes in female cells, is increased in women with recurrent pregnancy loss,90,91,92,93 and 94 although this finding was not confirmed in two studies.95,96 This observation has prompted speculation that X-linked mutations lethal to the male cause skewed X chromosome inactivation in female carriers and predispose to abortion of male conceptuses and an increased prevalence of female live births.92,97 However, investigation of this issue has not confirmed the predicted excess of chromosomally normal male abortuses.93 Observations of an increased prevalence of trisomic abortuses among women with recurrent pregnancy loss and skewed X chromosome inactivation have suggested the alternative hypothesis that X chromosome mutations or X-autosome translocations result in skewed X chromosome inactivation and a smaller than normal ovarian follicular pool or accelerated follicular depletion that predisposes to oocyte aneuploidy and recurrent pregnancy loss.93,98
Ill-fated chromosomally abnormal conceptuses can also result from fertilization of a normal euploid oocyte by an aneuploid sperm. The sperm of men whose partners have a history of unexplained recurrent pregnancy loss exhibit a higher prevalence of abnormal morphology, chromosome aneuploidy, DNA fragmentation, and abnormal tests of sperm
function such as hypo-osmotic swelling.99,100,101,102,103,104,105 and 106 The incidence of sperm aneuploidy rises with paternal age, if only slightly,58,107 and the incidence of miscarriage in young women with older male partners is higher than in those whose partners are young.108 Taken together, these observations suggest that poor semen quality, like a low ovarian reserve in women, can predispose to both infertility and early pregnancy loss, two different points on a continuum of reproductive failure having some causes in common. However, sperm aneuploidy rarely rises above approximately 1-2%. Compared to the influence of oocyte aneuploidy on miscarriage risk, chromosomally abnormal sperm have relatively little importance as a predisposing factor in recurrent pregnancy loss.
function such as hypo-osmotic swelling.99,100,101,102,103,104,105 and 106 The incidence of sperm aneuploidy rises with paternal age, if only slightly,58,107 and the incidence of miscarriage in young women with older male partners is higher than in those whose partners are young.108 Taken together, these observations suggest that poor semen quality, like a low ovarian reserve in women, can predispose to both infertility and early pregnancy loss, two different points on a continuum of reproductive failure having some causes in common. However, sperm aneuploidy rarely rises above approximately 1-2%. Compared to the influence of oocyte aneuploidy on miscarriage risk, chromosomally abnormal sperm have relatively little importance as a predisposing factor in recurrent pregnancy loss.
Karyotyping the Abortus
Many view karyotyping the products of conception following miscarriage as an unnecessary and expensive luxury. Others consider it crucially important for differentiating couples who are candidates for thorough evaluation from those who are not. Without karyotyping, women who repeatedly miscarry generally are assumed to be losing normal pregnancies when, in fact, most are not. Some have even advocated karyotyping the first or second abortus, reasoning that women who abort chromosomally normal pregnancies should be screened for treatable causes of pregnancy loss sooner rather than later. Conversely, those who miscarry a chromosomally abnormal pregnancy might be spared unnecessary and costly evaluation and empiric treatments.9
Unfortunately, the karyotype of an abortus cannot provide information so definitive; a karyotype may be useful but has limitations and pitfalls that must be carefully considered. Most early failed pregnancies lose viability well before onset of clinical symptoms of miscarriage or other recognition of the inevitable loss; products of such conceptions may therefore fail to grow in culture. Tissue specimens passed spontaneously are more likely to fail culture than those obtained by curettage.45
A normal abortus karyotype might be interpreted as suggesting that genetic factors are not likely responsible, focusing attention on evaluation for other possible causes of recurrent pregnancy loss.9 Unfortunately, a normal 46,XX karyotype can also represent maternal cell contamination of the tissue specimen and preferential growth of the normal maternal cell line in culture, particularly when no specific care has been taken to dissect, isolate, and submit only chorionic villi for cell culture.38,39 and 40 Results from an embryoscopic and cytogenetic study of early missed abortions challenge directly the notion that a normal karyotype effectively excludes genetic causes for a failed pregnancy. Whereas 75% of the abortuses were chromosomally abnormal, fully two-thirds of the remaining 25% having a normal karyotype (17% of the total) exhibited gross developmental abnormalities as severe as those observed in aneuploid abortuses.42 These observations strongly suggest that over 90% of all early missed abortions involving a recognizable embryo result from genetic errors and imply that a substantial proportion of failed early pregnancies result from gross genetic flaws in organizational and morphogenic processes not detectable with conventional cytogenetic techniques or even more modern methods (FISH, comparative genomic hybridization). Arguably, pregnancies that fail before any recognizable embryogenesis (empty sacs or “blighted ova”) are even more likely to result from chromosomal abnormalities, the inference being that well more than 90% of all early pregnancy losses can result from genetic causes.
An abnormal abortus karyotype revealing trisomy, monosomy, or polyploidy explains that specific pregnancy loss, suggests it likely resulted from chance alone, and generally has been considered as evidence that the risk for recurrence is not significantly increased.24,48 Whereas such findings may suggest that no formal evaluation is therefore needed, one must
assume that couples with other specific causes for recurrent pregnancy loss have at least the same random chance for conceiving an aneuploid pregnancy as anyone else; they might be overlooked if evaluation is offered only to those having a chromosomally normal abortus. Moreover, an aneuploid abortus might also reflect the influence of advanced maternal age or an otherwise unsuspected diminished ovarian reserve, in which case the risk for recurrence in a subsequent pregnancy clearly is increased.84
assume that couples with other specific causes for recurrent pregnancy loss have at least the same random chance for conceiving an aneuploid pregnancy as anyone else; they might be overlooked if evaluation is offered only to those having a chromosomally normal abortus. Moreover, an aneuploid abortus might also reflect the influence of advanced maternal age or an otherwise unsuspected diminished ovarian reserve, in which case the risk for recurrence in a subsequent pregnancy clearly is increased.84
A karyotype of an abortus that demonstrates an unbalanced chromosomal translocation obviously suggests that a parent may be a balanced carrier of the same translocation, a suspicion easily confirmed by performing karyotypes on both partners in affected couples.
The more expensive methods for genetic screening to detect variants associated with miscarriages, FISH and comparative genomic hybridization discussed below, can be applied to couples having recurrent miscarriages. However, these are expensive tests, and although variants and polymorphisms can be detected, the chances of having a healthy child, despite a higher risk of miscarriage, are still high, perhaps as high as non-carrier couples.109
Preimplantation Genetic Diagnosis and Aneuploidy Screening
Preimplantation genetic diagnosis describes a number of techniques for preconceptional genetic evaluation of embryos resulting from in vitro fertilization (IVF). Preimplantation genetic diagnosis can be used to detect numerical (aneuploidy) and structural chromosomal abnormalities (translocation, inversions), to identify oocytes or embryos with inherited single gene disorders (cystic fibrosis, thalassemia, hemophilia, Duchenne muscular dystrophy, and numerous others),110,111 or to determine gender.112,113 The technique requires one or more cells that may be obtained at different stages of development. The chromosomal composition of the oocyte may be inferred from that of the extruded polar bodies.110 One or two blastomeres may be removed from cleavage stage embryos. Biopsy of the trophoectoderm can also be performed at the blastocyst stage. In the most common scenario (cleavage stage embryo biopsy), a laser or a dilute solution of acid Tyrode’s solution is used to create a small hole in the zona pellucida and one or two cells are aspirated, typically on the third day after oocyte retrieval and fertilization when embryos are at the 6-8 cell stage.113
FISH, fluorescence in situ hybridization, is a technique for detection of numerical chromosomal abnormalities using probes labeled with different colored fluorochromes that bind to specific gene sequences on specific chromosomes. In the context of recurrent pregnancy loss, it has been used to screen embryos resulting from IVF for the most common aneuploidies observed in abortuses (XY, 13, 14, 15, 16, 18, 21, 22) and also to distinguish chromosomally normal, balanced, and unbalanced embryos in couples who carry a balanced chromosomal translocation.113,114 and 115 As a method for aneuploidy screening, FISH has both advantages and limitations. FISH is relatively easy to perform and yields results in time for transfer of genetically selected embryos 2 days after embryo biopsy (5 days after oocyte retrieval and fertilization), at the blastocyst stage. Although it allows evaluation of only a limited number of chromosomes (typically between 5 and 9), FISH can still detect over 80% of all chromosomal abnormalities because it typically includes all of the chromosomes involved in most aneuploidies.116 Because probes hybridize to a specific locus or the centromere, FISH provides information only about the presence or absence of a very small segment of the chromosome; partial aneuploidies can go undetected.117 Also, nuclear fragmentation in biopsied blastomeres is relatively common and can result in lost chromosomes, yielding erroneous diagnoses of aneuploidy.118
Comparative genomic hybridization is a related technique in which test DNA (extracted from a single blastomere) and normal male reference DNA (extracted from lymphocytes) are first amplified, then labeled with different colored fluorochromes (green/red), and simultaneously hybridized to template metaphase chromosomes from normal male lymphocytes; the green/red fluorescence ratio reflects the relative copy number for each chromosome in test DNA compared to the normal reference DNA.117 Comparative genomic hybridization allows analysis of all 24 chromosomes (X, Y, 22 autosomes) and detection of abnormalities not recognized by more limited analysis with FISH.118,119 This technique has been applied to the genetic evaluation of fetal losses; the diagnostic yield is improved compared with conventional karyotyping, which is often handicapped by culture failures or maternal contamination.120,121
Regardless whether FISH or comparative genomic hybridization is employed, preimplantation genetic diagnosis generally involves the analysis of only one or two blastomeres, assuming that those selected accurately represent the entire embryo. Unfortunately, a number of studies have now demonstrated that mosaicism is extremely common in early human embryos cultured in vitro. The prevalence of embryonic mosaicism increases with maternal age and with the stage of development; approximately half of all cleavage stage embryos and up to 90% of blastocysts exhibit some degree of chromosomal mosaicism.122,123,124,125 and 126 Diagnostic errors, therefore, are inevitable and, to some extent, unavoidable, but can be minimized by analyzing two or even three blastomeres.126,127
Overall, the results of preimplantation genetic diagnosis studies using both FISH and comparative genomic hybridization indicate that only approximately 35-45% of embryos are normal for all of the chromosomes examined.128,129,130 and 131 Data derived from numerous studies reveal that older women and women with a history of recurrent pregnancy loss produce more aneuploid embryos than younger women and those with normal reproductive histories.132,133,134,135 and 136 Transfer of preimplantation genetic diagnosis-selected embryos can improve implantation rates and decrease abortion rates in women at higher risk for pregnancy loss.126,127 and 128,137,138 The ultimate impact of preimplantation genetic diagnosis for aneuploidy screening on the live birth rate in older women and in women with history of recurrent pregnancy loss is not yet clear, although one cost/benefit analysis concluded that IVF alone is the most cost-effective option under the age of 40, but over age 40, IVF alone and IVF with preimplantation genetic diagnosis are equal in cost.139 Whereas it may be reasonable to consider preimplantation genetic diagnosis aneuploidy screening for indications of advanced maternal age or recurrent pregnancy loss in couples with other specific indications for IVF, the results achieved with preimplantation genetic diagnosis so far do not justify IVF with preimplantation genetic diagnosis for all couples with advanced maternal age or history of recurrent pregnancy loss. Up to now, the overwhelming majority of preimplantation genetic diagnosis has been performed in a very few centers worldwide, but further improvements in the technologies and wider application of the techniques are likely.
For couples with recurrent pregnancy loss in whom one partner carries a balanced chromosomal translocation, IVF with preimplantation genetic diagnosis and transfer of only normal and balanced embryos can achieve pregnancy rates comparable to those observed in unselected infertile couples with substantially decreased risk of spontaneous miscarriage, although pregnancy rates are inversely proportional to the proportion of abnormal gametes.115,140,141,142,143 and 144 When the male partner carries the balanced translocation, sperm FISH analysis can be used to determine the proportion of chromosomally unbalanced sperm and to predict the probability of conceiving a successful pregnancy.60 Data suggest that when there are numerous good-quality embryos and less than approximately 65% unbalanced sperm, translocation carrier couples have a reasonable probability of success with IVF and preimplantation genetic diagnosis, but otherwise not.60 Sperm FISH analysis could prove valuable to affected couples weighing the options of IVF with preimplantation genetic diagnosis and therapeutic insemination with donor sperm. Unfortunately, there is no way
to obtain similar information for female balanced translocation carriers; depending on the nature of the translocation and on the reproductive history, some women who carry a balanced translocation may prefer to apply their available resources to IVF with donor oocytes rather than attempt IVF with preimplantation genetic diagnosis.
to obtain similar information for female balanced translocation carriers; depending on the nature of the translocation and on the reproductive history, some women who carry a balanced translocation may prefer to apply their available resources to IVF with donor oocytes rather than attempt IVF with preimplantation genetic diagnosis.
Summary of Key Facts Relating to Genetic Factors
Overall, 50-75% of spontaneous miscarriages result from numerical chromosomal abnormalities in the embryo or fetus and occur by chance; trisomies are the most common. In approximately 5% of couples with recurrent pregnancy loss, karyotypes will reveal a balanced chromosomal translocation that markedly increases the risk of miscarriage due to the high prevalence of aneuploidy in the gametes of the affected parent. Reproductive aging in women is associated with an increasing risk of miscarriage, which reflects a rising prevalence of oocyte aneuploidy. Ovarian reserve testing in women with unexplained recurrent pregnancy loss can reveal evidence of premature reproductive aging. Karyotype of an abortus can explain the loss (aneuploidy), provide evidence for a chromosomal translocation in a parent (when an unbalanced translocation is observed), or suggest a non-genetic cause (when normal). However, a normal karyotype does not entirely exclude genetic causes for the miscarriage, and a normal female karyotype (46,XX) can result from maternal cell contamination of cultured tissue specimens. IVF with preimplantation genetic diagnosis and selected transfer of euploid embryos is an established treatment for couples with recurrent pregnancy loss when one partner carries a balanced chromosomal translocation. IVF with preimplantation genetic diagnosis (using FISH) for reasons of advanced maternal age or in couples with unexplained recurrent pregnancy loss can increase implantation rates and decrease miscarriage risk, but have not increased live birth rates. Consequently, the associated costs in couples without other specific indications for IVF cannot be justified.
Anatomic Factors
The anatomic uterine abnormalities that can predispose to a higher risk of pregnancy loss include congenital malformations, uterine leiomyomas, and intrauterine adhesions. Each has been considered in detail elsewhere as factors that may also adversely affect fertility (Chapters 4 and 27); discussion here is limited to their importance and management in women with a history of recurrent pregnancy loss.
The principal methods for evaluation of the uterus include traditional hysterosalpingography (HSG), transvaginal ultrasonography, and sonohysterography. Magnetic resonance imaging (MRI) and endoscopy (hysteroscopy and laparoscopy) are generally reserved when necessary for better defining the nature of anomalies identified or suggested by simpler methods. Each method and its limitations, pitfalls, and relative accuracy have been described at length in the context of the infertility evaluation (Chapter 27). However, their relative value for the evaluation of infertility and recurrent pregnancy loss are somewhat different. HSG has some advantages over ultrasonographic techniques for the evaluation of uterine factors in infertile women because it also provides information about tubal patency that transvaginal ultrasonography and sonohysterography cannot. However, for the evaluation of recurrent pregnancy loss, transvaginal ultrasonography and sonohysterography offer distinct advantages over HSG; both image the external uterine fundal contour
and, therefore, better distinguish septate and bicornuate uteri,145,146 and 147 and both are generally easier to perform and better tolerated than HSG. Compared to HSG, sonohysterography has greater sensitivity and specificity for detection of intracavitary mass lesions (submucous myomas, endometrial polyps) and similar efficacy for diagnosis of intrauterine adhesions.148,149,150,151,152 and 153 Three dimensional transvaginal ultrasonography, with and without saline contrast, can provide high-definition images comparable to those generated with MRI.154,155 As in women with infertility, endoscopic methods can usually be reserved for excision of cavitary mass lesions or intrauterine septa identified by simpler methods.
and, therefore, better distinguish septate and bicornuate uteri,145,146 and 147 and both are generally easier to perform and better tolerated than HSG. Compared to HSG, sonohysterography has greater sensitivity and specificity for detection of intracavitary mass lesions (submucous myomas, endometrial polyps) and similar efficacy for diagnosis of intrauterine adhesions.148,149,150,151,152 and 153 Three dimensional transvaginal ultrasonography, with and without saline contrast, can provide high-definition images comparable to those generated with MRI.154,155 As in women with infertility, endoscopic methods can usually be reserved for excision of cavitary mass lesions or intrauterine septa identified by simpler methods.
Congenital Uterine Malformations
Developmental uterine anomalies have long been associated with pregnancy loss and obstetric complications. The reported prevalence of uterine malformations in women with recurrent pregnancy loss has varied widely with differences in diagnostic methods and criteria.156,157 and 158 The best available data suggest that the prevalence of major uterine anomalies (excluding arcuate uteri) in the general population is approximately 2% and about three times greater (6-7%) in women with history of recurrent pregnancy loss, supporting the notion that uterine malformations may indeed be the proximate cause for miscarriages in a small proportion of women with recurrent pregnancy loss.156,159,160,161 and 162 Pregnancy losses with uterine congenital abnormalities usually occur later in pregnancy in the second trimester; however, the presence of an uterine anomaly after repeated early losses deserves consideration of surgical repair.163,164 The pathogenesis of pregnancy wastage in women with congenital uterine malformations is uncertain but generally has been attributed to a reduced intrauterine volume or poor vascular supply.165
A unicornuate uterus results from failure of development of one müllerian duct. Pregnancy outcomes in women with unicornuate uteri are generally poor; approximately half of all recognized pregnancies fail.166 Most unicornuate uteri are associated with a noncommunicating contralateral uterine horn, some of which have a functional cavity and should be removed to reduce risk for ectopic pregnancy, even if not otherwise necessary (pain, mass, endometriosis).167 Since approximately 40% of unicornuate uteri are associated with an ipsilateral renal agenesis, further evaluation with an intravenous pyelogram or renal sonogram is also indicated.167 No surgical procedure can enlarge the unicornuate uterus. Anecdotal reports of successful pregnancies after cervical cerclage are numerous, but the efficacy of cerclage in women with unicornuate uteri has not been carefully studied. Available evidence suggests that most pregnancies in women with unicornuate uteri are best managed expectantly with cervical cerclage reserved for those with previous second trimester pregnancy losses or evidence of progressive cervical shortening.166
Uterine didelphys results from complete failure of fusion of the müllerian ducts and normal differentiation of each to form a cervix and hemiuterus. The reproductive outcomes of women with uterine didelphys are slightly better than those of women with unicornuate uteri, possibly because of improved collateral blood supply between the two fused horns. Nevertheless, approximately 40% of pregnancies in women with uterine didelphys end in spontaneous miscarriage.166 In general, the only surgery indicated in women with uterine didelphys is the removal of an obstructing longitudinal vaginal septum (75% prevalence).168 Unification procedures are usually unnecessary and meddlesome, but can benefit some women with numerous miscarriages or previable births. When surgery is performed, the recommended technique unifies the two fundi and leaves the two cervices intact.169
A bicornuate uterus results from incomplete fusion of the müllerian ducts at the level of the fundus, creating two separate uterine cavities with a common lower segment and a single cervix; externally, the uterus has a midline cleft with a depth that varies with the severity of the fusion anomaly. Data from collected series of women with bicornuate uteri reveal miscarriage and overall fetal loss rates of approximately 30% and 40%, respectively.166 Preterm delivery risks decrease as the size of the common lower uterine cavity increases.170 Although the benefits of unification procedures have not been systematically evaluated, surgery generally is considered unnecessary and best reserved for those with a well established history of otherwise unexplained recurrent pregnancy loss or previable births.166 The Strassman abdominal metroplasty is the surgical procedure of choice and comparisons between pregnancy outcomes before and after unification suggest that surgery can benefit carefully selected women.171,172 The incidence of cervical incompetence associated with congenital uterine anomalies is reportedly highest for those having a bicornuate uterus, and there is evidence from case series that cervical cerclage can improve fetal survival rates.173,174
A septate uterus results from incomplete resorption of the medial septum separating the two otherwise normally fused hemiuteri. Septum resorption normally occurs only after urologic development is completed; the prevalence of urinary tract anomalies, therefore, is not increased in women with septate uteri. The septate uterus is by far the most common uterine developmental anomaly, accounting for 80-90% of all major malformations in both women with recurrent pregnancy loss (3.5% prevalence) and in the general population.145,160,162,175 It is also the malformation most highly associated with poor pregnancy outcomes.145,176 Data from numerous case series indicate that the miscarriage rate associated with septate uteri is approximately 65%.166 Uterine septa associated with recurrent pregnancy loss are not broader or longer than those observed in women with normal reproductive histories. However, the size of the unaffected cavity (bounded by the leading edge of the septum above and the internal cervical os below) is smaller in women with recurrent pregnancy loss,156 an observation lending credence to the hypothesis that implantation on a poorly vascularized septum predisposes to pregnancy loss.177,178 and 179 Although uterine septa are not always associated with a poor pregnancy outcome, their discovery in women with recurrent pregnancy loss provides an indication for surgical correction. Hysteroscopic septoplasty is a relatively brief and straightforward outpatient endoscopic procedure associated with low morbidity and dramatically improved postoperative pregnancy outcomes (80% term delivery, 5% preterm delivery, 15% pregnancy loss).145,166 Hysteroscopic septoplasty can be performed using microscissors, any of a variety of electrosurgical instruments, or laser, and excellent results can be achieved with all methods.145 With few exceptions, only incision rather than excision is required because the septum typically retracts, leaving little if any residual. The procedure usually is complete when both tubal ostia can be viewed at the same time. It is useful to remember that an arcuate uterus is generally regarded as a normal variant and that residual septa measuring less than 1 cm in size have no adverse effect on pregnancy outcome.180
Nearly 70% of women exposed to diethylstilbestrol (DES) in utero have a developmental uterine abnormality.181 The T-shaped uterine cavity is the single most common malformation; others include a hypoplastic uterus, constriction rings, and irregular intrauterine filling defects. Although the use of DES in pregnancy was banned in 1971 because of an observed association with vaginal clear cell adenocarcinoma and most exposed women are now beyond their reproductive years,182 affected women are still occasionally encountered. In utero DES-exposed women are at increased risk of adverse pregnancy outcomes, including a 2-fold higher risk of spontaneous miscarriage (approximately 24%) and a dramatic 9-fold higher risk of ectopic pregnancy.183 Structural changes in cervical collagen content may predispose affected women to cervical incompetence, and data from nonrandomized clinical trials suggest cerclage warrants serious consideration in women with history of second trimester loss or preterm delivery.184,185
Uterine Leiomyomas
There is no substantial evidence implicating uterine myomas as a cause of recurrent pregnancy loss. What evidence does exist derives from case series comparing reproductive outcomes before and after myomectomy.186,187 All of the mechanisms proposed to explain how myomas might predispose to recurrent pregnancy loss relate to the consequences of poor regional blood flow.188 Numerous studies have examined the effect of uterine fibroids on fertility (Chapters 4 and 27), but none has specifically examined the effect of myomas on pregnancy outcome infertile women. The best available data come from a series of studies designed to examine the effect of uterine myomas on outcomes achieved with in vitro fertilization (IVF) in infertile women. On balance, these data suggest that pregnancy outcomes, like pregnancy and implantation rates, are adversely affected by submucous myomas, but not by subserosal or intramural myomas under 5-7 cm in size.189,190,191 and 192 Consequently, management recommendations for women with recurrent pregnancy loss and uterine myomas are comparable to those for infertile women with uterine myomas.
In general, when submucous myomas are single and small, the likely benefits of hysteroscopic myomectomy outweigh the few associated risks.193,194,195,196 and 197 When submucous myomas are multiple or large, hysteroscopic myomectomy is more technically challenging and has greater risks, including sterility resulting from severe postoperative intrauterine adhesions. When submucous myomas extend deeply into the myometrium, treatment options include subtotal hysteroscopic myomectomy and abdominal myomectomy. When myomas have definite but limited impact on the uterine cavity, the decision to delay or to proceed with surgical treatment may vary, depending on age, reproductive history, size and location of myomas, and the complexity of any other treatments required. When myomas do not encroach on or distort the uterine cavity, surgery is not indicated in the absence of other specific symptoms attributable to myomas that demand treatment in and of themselves.
Intrauterine Adhesions (Asherman’s Syndrome)
Recurrent pregnancy loss is one possible result of intrauterine adhesions, but menstrual disorders (hypomenorrhea, amenorrhea, dysmenorrhea) and infertility are the more common clinical presentations.198,199 Any insult severe enough to remove or destroy endometrium can cause intrauterine adhesions, and the gravid uterus seems particularly susceptible to injury.200,201 Considering that pregnancy loss is among the most common indications for uterine curettage, intrauterine adhesions can first result from and then become a contributing cause of recurrent pregnancy loss. Mechanisms by which intrauterine adhesions can cause recurrent pregnancy loss include a decreased functional intrauterine volume and endometrial fibrosis and inflammation that can predispose to placental insufficiency.172 The pregnancy outcomes of women with intrauterine adhesions are generally poor (40-80% ending in spontaneous miscarriage and approximately 25% in preterm delivery) and much improved after adhesiolysis (50-90% ending in term delivery, 7-23% ending in miscarriage); the prognosis generally correlates with the severity of disease.199,200,202,203,204 and 205
Hysteroscopy provides the means to confirm a diagnosis of intrauterine adhesions suggested by sonohysterography or HSG and is also the method of choice for treatment, being both safer and more effective than blind curettage. The hysteroscopic appearance of adhesions of varying severity, techniques for lysis, operative risks, and adjuvant therapies are described in detail in Chapter 27.
Summary of Key Facts Relating to Anatomic Factors
Congenital and acquired uterine abnormalities predispose to an increased risk of pregnancy loss and can be identified by sonohysterography or traditional HSG; magnetic resonance imaging may be required to accurately differentiate septate and bicornuate uteri. The septate uterus is the most common müllerian anomaly, the one most closely correlated with pregnancy loss, and the malformation most easily and successfully corrected; hysteroscopic septoplasty is indicated in women with recurrent pregnancy loss and having a septate uterus. Abdominal metroplasty procedures are rarely indicated for women with a uterus didelphys or a bicornuate uterus. Cervical cerclage may help to improve pregnancy outcomes in women with bicornuate uteri and in those with a unicornuate uterus or a uterus didelphys who have a history of previable delivery or exhibit progressive cervical shortening during early pregnancy. The prevalence of urinary tract abnormalities is increased in women having a unicornuate or bicornuate uterus or a uterus didelphys, but not in those having a septate uterus. Uterine leiomyomas are often identified in women with recurrent pregnancy loss, but only submucous myomas and larger intramural fibroids that clearly encroach upon or displace the uterine cavity are relevant. Intrauterine adhesions are an uncommon but established cause of recurrent miscarriage; pregnancy outcomes are much improved after hysteroscopic lysis.
Immunologic Factors
Both autoimmune and alloimmune mechanisms have been implicated as causes of recurrent pregnancy loss. Autoimmune disorders involve an immune response directed against a specific part of the host or self; those that have been linked to recurrent pregnancy loss include certain classic autoimmune diseases like systemic lupus erythematosus and the antiphospholipid syndrome. Alloimmune disorders involve an abnormal maternal immune response to fetal or placental antigens; possibilities include maternal cytotoxic antibodies, absent maternal blocking antibodies, and disturbances in natural killer cell function and distribution.
Autoimmune Disorders
Systemic lupus erythematosus has long been associated with pregnancy loss. Data from a number of case series suggest that the risk for loss is approximately 20%, all excess risk being attributable to losses occurring during the second and third trimester of pregnancy.206 Early spontaneous miscarriages are no more common in women with systemic lupus than in the general population, but the incidence of later losses (6%) is two to four times higher.207 Almost all fetal deaths that occur in women with systemic lupus are associated with antiphospholipid antibodies; they are the most sensitive indicator of fetal distress or death.208,209 and 210 Active disease at conception, onset of systemic lupus erythematosus during pregnancy, and renal disease also increase the risk for pregnancy loss.207 Careful monitoring and timely interventions can improve pregnancy outcomes.211,212 Treatment aimed at preventing pregnancy loss in women with systemic lupus and antiphospholipid antibodies is similar to that for women with antiphospholipid syndrome, discussed later. In general, women with active systemic lupus erythematosus should be advised to delay conception until remission can be established, those with moderate renal insufficiency must be advised
of the increased risk for pregnancy loss, and women with severe renal insufficiency should be encouraged to avoid pregnancy; even successful pregnancies are at increased risk for preeclampsia and preterm delivery.207
of the increased risk for pregnancy loss, and women with severe renal insufficiency should be encouraged to avoid pregnancy; even successful pregnancies are at increased risk for preeclampsia and preterm delivery.207
Antiphospholipid syndrome is an autoimmune disorder having specific clinical and laboratory features; diagnosis requires at least one of each.213,214 The clinical diagnostic criteria include thromboembolic events (arterial, venous, small vessel) and pregnancy loss (three or more consecutive losses at less than 10 weeks’ gestation, a fetal death after 10 weeks, premature birth at less than 34 weeks associated with severe preeclampsia or placental insufficiency). There are also three laboratory diagnostic criteria. One is the lupus anticoagulant, revealed by delayed clotting in phospholipid-dependent coagulation tests (activated partial thromboplastin time, kaolin clotting time, dilute Russell’s viper venom time), corrected by addition of excess phospholipid but not by platelet-poor plasma.215 The second is the demonstration of moderate to high levels of anticardiolipin antibodies (IgG or IgM); low levels may be observed in 3-5% of normal individuals and are of uncertain significance.216 More recently, a high titer of antibodies to β2-glycoprotein 1 is also considered sufficient to establish the diagnosis.217 Abnormal laboratory test results must be observed on at least two separate occasions at least 12 weeks apart.
International Consensus Definition for the Diagnosis of Antiphospholipid Syndrome218
DIAGNOSIS REQUIRES ONE OF THE CLINICAL CRITERIA AND ONE OF THE LABORATORY FINDINGS
Clinical Criteria:
Vascular Thrombosis
Pregnancy Morbidity
One or more losses after the 10th week of a morphologically normal fetus.
One or more premature births of a normal neonate before the 34th week because of preeclampsia or eclampsia or placental insufficiency.
Three or more unexplained consecutive early miscarriages.
Laboratory Tests:
Lupus anticoagulant present on two or more occasions at least 12 weeks apart.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


