Neuroendocrinology
 |
There are two major sites of action within the brain that are important in the regulation of reproductive function—the hypothalamus and the pituitary gland. In the past, the pituitary gland was viewed as the master gland. Then a new concept emerged in which the pituitary was relegated to a subordinate role as part of an orchestra, with the hypothalamus as the conductor, responding to both peripheral and central nervous system messages and exerting its influence by means of neurotransmitters transported to the pituitary by a portal vessel network. Regardless of which site was dominant, the conventional thesis was that the central nervous system-pituitary complex determined and directed the chronology of developmental events within a responsive ovary. However, there is now a new concept— the complex sequence of events known as the menstrual cycle is controlled by the sex steroids and peptides produced within the very follicle destined to ovulate. The hypothalamus and its direction, and the pituitary, are essential for the operation of the entire mechanism, but the endocrine function that leads to ovulation is brought about by endocrine feedback on the anterior pituitary.
A full understanding of this feature of reproductive biology will benefit the clinician who faces problems in gynecologic endocrinology. With this understanding, the clinician can comprehend the hitherto mysterious but significant effects of stress, diet, exercise, and other diverse influences on the pituitary-gonadal axis. Furthermore, we will be prepared to make advantageous use of the numerous neuropharmacologic agents that are the dividends of neuroendocrine research. To these ends, this chapter offers a clinically oriented review of the current status of reproductive neuroendocrinology.
Hypothalamic-Hypophyseal Portal Circulation
The hypothalamus is at the base of the brain just above the junction of the optic nerves. In order to influence the anterior pituitary gland, the brain requires a means of transmission or connection. A direct nervous connection does not exist. The blood supply of the anterior pituitary, however, originates in the capillaries that richly lace the median eminence area of the hypothalamus. The superior hypophyseal arteries form a dense network of capillaries within the median eminence, which then drain into the portal vessels that descend along the pituitary stalk to the anterior pituitary. The direction of the blood flow in this hypophyseal portal circulation is from the brain to the pituitary. Section of the neural stalk, which interrupts this portal circulation, leads to inactivity and atrophy of the gonads, along with a decrease in adrenal and thyroid activity to basal levels. With regeneration of the portal vessels, anterior pituitary function is restored. Thus, the anterior pituitary gland is under the influence of the hypothalamus by means of neurohormones released into this portal circulation. There also exists retrograde flow so that pituitary hormones can be delivered directly to the hypothalamus, creating the opportunity for pituitary feedback on the hypothalamus. An additional blood supply is provided by short vessels that originate in the posterior pituitary that in turn receives its arterial supply from the inferior hypophyseal arteries.
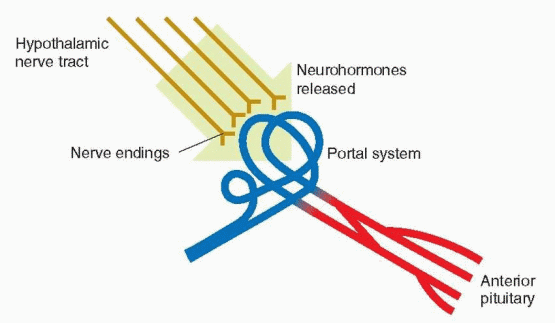 |
The Neurohormone Concept
The influence of the pituitary by the hypothalamus is achieved by materials secreted in the cells of the hypothalamus and transported to the pituitary by the portal vessel system. Indeed, pituitary cell proliferation and gene expression are controlled by hypothalamic peptides and their receptors. In addition to the stalk section experiments cited previously, transplantation of the pituitary to ectopic sites (e.g., under the kidney capsule) results in failure of gonadal function. With retransplantation to an anatomic site under the median eminence, followed by regeneration of the portal system, normal pituitary function is regained. This retrieval of gonadotropic function is not accomplished if the pituitary is transplanted to other sites in the brain. Hence, there is something very special about the blood draining the basal hypothalamus. An exception to this overall pattern of positive influence is the control of prolactin secretion. Stalk secretion and transplantation cause release of prolactin from the anterior pituitary, implying a negative, inhibitory hypothalamic control. Furthermore, cultures of anterior pituitary tissue release prolactin in the absence of hypothalamic tissue or extracts.
 |
Neuroendocrine agents originating in the hypothalamus have positive stimulatory effects on growth hormone, thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), as well as the gonadotropins, and represent the individual neurohormones of the hypothalamus. The neurohormone that controls gonadotropins is called gonadotropin-releasing hormone (GnRH). The neurohormone that controls prolactin is called prolactininhibiting hormone and is dopamine. Human corticotropin-releasing hormone (CRH) is a 41 amino acid peptide that is the principal regulator of ACTH secretion, and that also activates the sympathetic nervous system. As we shall see, CRH can suppress gonadotropin secretion, an action partly mediated by endorphin inhibition of GnRH.
In addition to their effects on the pituitary, behavioral effects within the brain have been demonstrated for several of the releasing hormones. Thyrotropin-releasing hormone (TRH) antagonizes the sedative action of a number of drugs and also has a direct antidepressant effect in humans. GnRH evokes mating behavior in male and female animals.1
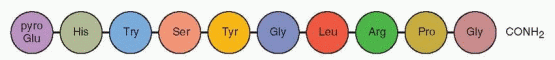
Initially, it was believed that there were two separate releasing hormones, one for folliclestimulating hormone (FSH) and another for luteinizing hormone (LH). It is now accepted that there is a single neurohormone (GnRH) for both gonadotropins. GnRH is a small peptide with 10 amino acids with some variation in the amino acid sequence among various mammals. Purified or synthesized GnRH stimulates both FSH and LH secretion. The divergent patterns of FSH and LH in response to a single GnRH are due to the modulating influences of the endocrine environment, specifically the feedback effects of steroids on the anterior pituitary gland.
The classic neurotransmitters are secreted at the nerve terminal. Brain peptides require gene transcription, translation, and posttranslational processing, all within the neuronal cell body. The final product is transported down the axon to the terminal for secretion. Small neuroendocrine peptides share common large precursor polypeptides, called polyproteins or polyfunctional peptides. These proteins can serve as precursors for more than one biologically active peptide.
The gene that encodes for the 92 amino acid precursor protein for GnRH, pro-GnRH, is located on the short arm of chromosome 8.2 The precursor protein for GnRH contains (in the following order) a 23 amino acid signal sequence, the GnRH decapeptide, a 3 amino acid proteolytic processing site, and a 56 amino acid sequence called GAP (GnRH-associated peptide).3 GAP is a potent inhibitor of prolactin secretion as well as a stimulator of gonadotropins; however, a physiologic role for GAP has not been established.4 Its primary role may be to provide appropriate conformational support for GnRH.
It is now apparent that GnRH has autocrine-paracrine functions throughout the body. It is present in both neural and nonneural tissues, and receptors are present in many extrapituitary tissues (e.g., the ovarian follicle and the placenta). Although GnRH is identical in all mammals, other nonmammalian forms exist, indicating that the GnRH molecule has existed for at least 500 million years.5,6 The central sequence, Tyr-Gly-Leu-Arg, is the nonconserved segment of GnRH, the segment with the most variability in other species. Accordingly, substitutions in this segment are well tolerated.
A second form of GnRH, known as GnRH-II, has been known to exist in many other species. GnRH-II consists of the following sequence: pGln-His-Trp-Ser-His-Gly-Trp-Tyr-Pro-Gly.
Prompted by its existence in other species, a search for its presence in humans was ultimately successful. A gene encoding GnRH-II is located on the human chromosome 20p13, obviously distinct from the GnRH-I gene on 8p11.2-p21.7 Both genes produce a peptide with a signal sequence, a GnRH decapeptide, a proteolytic site, and a GAP. Human GnRH-II expression is highest outside the brain. An analysis of the evolution of GnRH indicates three major forms: GnRH localized to the hypothalamus (GnRH-I), forms in midbrain nuclei and outside the brain (GnRH-II), and forms in several fish species (GnRH-III), thus indicating appearance of the various forms before the emergence of vertebrates.7 A total of 24 forms of GnRH have been identified in multiple species, but GnRH-I and GnRH-II are the primary GnRHs in mammals.8 GnRH-I is the main form found in the brain, whereas GnRH-II is widely distributed in other organs.
Prompted by its existence in other species, a search for its presence in humans was ultimately successful. A gene encoding GnRH-II is located on the human chromosome 20p13, obviously distinct from the GnRH-I gene on 8p11.2-p21.7 Both genes produce a peptide with a signal sequence, a GnRH decapeptide, a proteolytic site, and a GAP. Human GnRH-II expression is highest outside the brain. An analysis of the evolution of GnRH indicates three major forms: GnRH localized to the hypothalamus (GnRH-I), forms in midbrain nuclei and outside the brain (GnRH-II), and forms in several fish species (GnRH-III), thus indicating appearance of the various forms before the emergence of vertebrates.7 A total of 24 forms of GnRH have been identified in multiple species, but GnRH-I and GnRH-II are the primary GnRHs in mammals.8 GnRH-I is the main form found in the brain, whereas GnRH-II is widely distributed in other organs.
A hypothalamic 12-amino acid peptide that inhibits pituitary secretion of gonadotropins was isolated from the brain of the Japanese quail.9 This peptide was identified in mammals as well and labeled gonadotropin-inhibitory hormone (GnIH), but its presence and possible role in primate reproduction remain to be determined.10
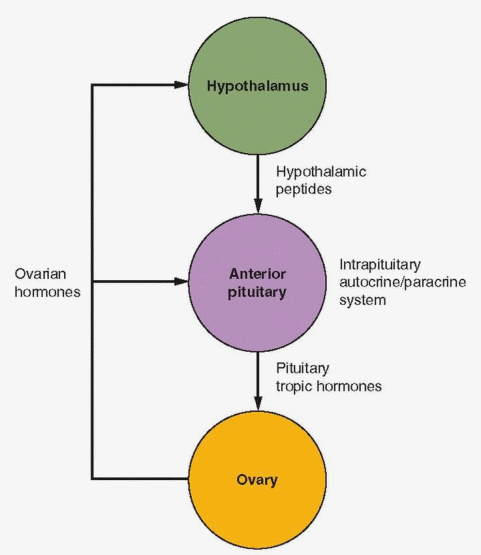
Perhaps the notion that the pituitary is a master gland should not be discarded. Although it is highly regulated by input from other sites, its function is essential for sustaining life. The hormones from the pituitary gland regulate puberty, growth, reproduction, metabolism, osmotic balance, and responses to stress. Pituitary development and activity are under the control of the hypothalamus (with input from other central nervous system sites), and pituitary response is finely tuned by hormonal messages from tissues that are the targets of the pituitary trophic hormones. In addition, the pituitary has its own auto-crine-paracrine system for enhancement and suppression of growth and function. But the pituitary gland is the focus for all of this activity, and this central, coordinating role is critical for normal life.
 |
Prolactin Secretion
Prolactin gene expression occurs in the lactotropes of the anterior pituitary gland, in decidualized endometrium, and in the myometrium. The prolactin secreted in these various sites is identical, but there are differences in mRNA indicating differences in prolactin gene regulation.
Transcription of the prolactin gene is regulated by a transcription factor (a protein named Pit-1) that binds to the 5′ promoter region and that is also necessary for growth hormone and TSH secretion.11,12 The expression of Pit-1 is in turn regulated by a transcription factor, Prop1 (Prophet of Pit-1); mutations in the Prop1 binding site in the Pit-1 gene result in defi-ciencies in multiple pituitary hormones.12,13 These mutations account for the majority of inherited cases of combined pituitary hormone deficiency. The remainder arise from mutations involving other transcription factors, specifically HESX1, LHX3, LHX4, TBX19, SOX2, AND SOX3.14
Prolactin gene transcription is regulated by the interaction of estrogen and glucocorticoid receptors with 5′ flanking sequences. Mutations in the sequences of these flanking regions or in the gene for the Pit-1 protein can result in the failure to secrete prolactin. The Pit-1 gene is also involved in differentiation and growth of anterior pituitary cells; therefore, mutations in this gene can lead not only to absent secretion of growth hormone, prolactin, and TSH but also to an absence of their trophic cells in the pituitary—the result is significant hypopituitarism.15 Molecular studies indicate that Pit-1 participates in mediating both stimulatory and inhibitory hormone signals for prolactin gene transcription. However, alterations in Pit-1 gene expression are not involved in pituitary tumor formation.16
The main function of prolactin in mammals is lactogenesis, whereas, in fish, prolactin is important for osmoregulation. The prolactin gene from the Chinook salmon contains coding sequences that are similar to those in mammals, and it is regulated similarly in the pituitary.17 Pit-1, the pituitary specific transcription factor, therefore, appears to be highly conserved among species. Sexual arousal and orgasm in men and women produce marked elevations of circulating levels of prolactin that persist for at least 1 hour, perhaps contributing to the suppression of sexuality immediately after arousal and orgasm.18,19
Prolactin gene expression is further regulated by other species-specific factors. Prolactin gene transcription is stimulated by estrogen and mediated by estrogen receptor binding to estrogen-responsive elements. This activation by estrogen requires interaction with Pit-1, in a manner not yet determined. Proximal promoter sequences are also activated by peptide hormones binding to cell surface receptors, e.g., TRH and growth factors. In addition, various agents that control cyclic AMP and calcium channels can stimulate or inhibit prolactin promoter activity.
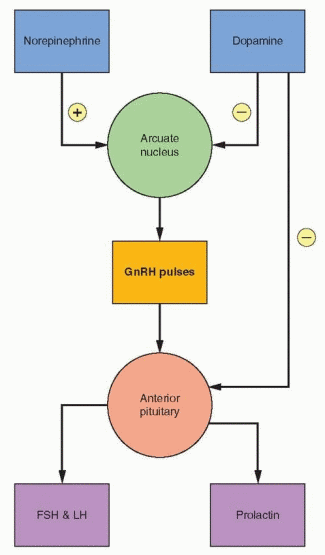
Pituitary secretion of prolactin is chiefly, if not totally, under the inhibitory control of hypo-thalamic dopamine released into the portal circulation, a tonic inhibition that requires a high output of dopamine.20 The action of dopamine in the pituitary is mediated by receptors that are coupled to the inhibition of adenylate cyclase activity. There are 5 forms of the dopamine receptor divided into 2 functional groups, D1 and D2, encoded by a single gene on chromosome 5 near the growth hormone receptor gene.21,22 The D2 type is the predominant receptor in the anterior pituitary gland. The structure and function of the dopamine receptors are of the G protein system described in Chapter 2. Binding of dopamine to the receptor leads to suppression of adenylate cyclase and cyclic AMP maintenance of prolactin gene transcription and prolactin secretion. Other mechanisms are also activated, including suppression of intracellular calcium levels. Pit-1 binding sites are involved in this
dopamine response. In addition to direct inhibition of prolactin gene expression, dopamine binding to the D2 receptor also inhibits lactotroph development and growth. These multiple effects of dopamine explain the ability of dopamine agonists to suppress prolactin secretion and the growth of prolactin-secreting pituitary adenomas. No activating or inactivating mutations of the dopamine receptors have been reported.
dopamine response. In addition to direct inhibition of prolactin gene expression, dopamine binding to the D2 receptor also inhibits lactotroph development and growth. These multiple effects of dopamine explain the ability of dopamine agonists to suppress prolactin secretion and the growth of prolactin-secreting pituitary adenomas. No activating or inactivating mutations of the dopamine receptors have been reported.
Several factors exert a stimulatory effect on prolactin secretion (prolactin-releasing factors), especially TRH, vasoactive intestinal peptide (VIP), epidermal growth factor, and perhaps GnRH. These factors interact with each other, affecting the overall lactotroph responsiveness; however, the only important clinical manifestation is the association of hyperprolactinemia with the elevation in TRH secretion that occurs with hypothyroidism. Prolactin homeostasis is regulated mainly by prolactin itself, feeding back on the dopamine-releasing neurons.
This dopaminergic mechanism is highly influenced by estrogen, either directly or through other neurotransmitters. Prolactin secretion can be understood by viewing dopamine received through the portal system as responsible for tonic inhibition. The dopaminergic system is stimulated by prolactin (decreasing secretion) and inhibited by estrogen (increasing secretion). Modulating influences include the inhibitory activity of endogenous opioids on dopamine release and stimulation by many substances, including serotonin and neuropeptide Y. Prolactin levels are highest during sleep.
Drugs used to treat psychological disorders are dopamine receptor antagonists. The inhibitory activity of dopamine on pituitary secretion of prolactin is blocked by these drugs, and prolactin levels increase in the circulation. Some of the newer drugs in this class do not affect prolactin secretion; these include clozapine, olanzapine, quetiapine, aripiprazole, and ziprasidone. Risperidone and amisulpride, however, act like the older drugs and increase prolactin secretion. The differences reflect the ability of each drug to cross the blood-brain barrier and the variations in affinity for the dopamine receptor.
The Hypothalamus and GnRH Secretion
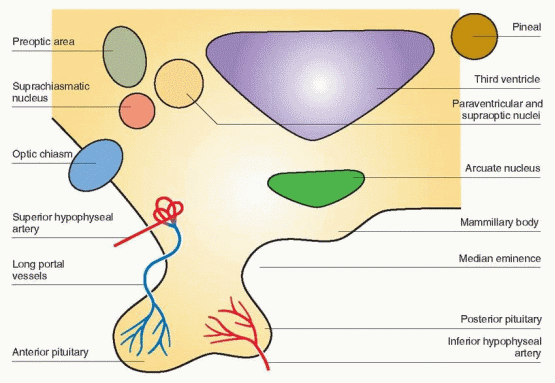
The hypothalamus is the part of the diencephalon at the base of the brain that forms the floor of the third ventricle and part of its lateral walls. Within the hypothalamus are peptidergic neural cells that secrete the releasing and inhibiting hormones. These cells share the characteristics of both neurons and endocrine gland cells. They respond to signals in the bloodstream, as well as to neurotransmitters within the brain in a process known as neurosecretion. In neurosecretion, a neurohormone or neurotransmitter is synthesized on the ribosomes in the cytoplasm of the neuron, packaged into a granule in the Golgi apparatus, and then transported by active axonal flow to the neuronal terminal for secretion into a blood vessel or across a synapse.
The cells that produce GnRH originate from the olfactory area. By migration during embryogenesis, the cells move along cranial nerves connecting the nose and the forebrain to their primary location, where eventually 1,000-3,000 GnRH-producing cells can be found in the arcuate nucleus of the hypothalamus extending their axons to the median eminence.23 The GnRH neurons appear in the medial olfactory placode (a thickened plate of ectoderm from which a sense organ develops) and enter the brain with the nervus terminalis, a cranial nerve that projects from the nose to the septal-preoptic nuclei in the brain.24 This amazing journey accounts for Kallmann’s syndrome, an association between an absence of GnRH and a defect in smell (a failure of both olfactory axonal and GnRH neuronal migration from the olfactory placode). Three modes of transmission have been documented: X-linked,
autosomal-dominant, and autosomal-recessive.25 The 5-7-fold increased frequency in males indicates that X-linked transmission is the most common. The mutations responsible for this syndrome result in the failure to produce proteins with functions that are necessary for neuronal migration, anosmin-1 in X-linked forms, a protein that is homologous to members of the fibronectin family and responsible for cell adhesion and protease inhibition, and the receptors for fibroblast growth factor and prokineticin in the autosomal forms.26,27 and 28 Normal GnRH neuron development and migration also depend on receptors that are tyrosine kinases, and abnormalites in these receptors might explain some clinical cases of GnRH deficiency.29 Like olfactory epithelial cells in the nasal cavity, GnRH neurons have cilia.30 The olfactory origin and the structural similarity of GnRH neurons and nasal epithelial cells suggest an evolution from reproduction controlled by pheromones.
autosomal-dominant, and autosomal-recessive.25 The 5-7-fold increased frequency in males indicates that X-linked transmission is the most common. The mutations responsible for this syndrome result in the failure to produce proteins with functions that are necessary for neuronal migration, anosmin-1 in X-linked forms, a protein that is homologous to members of the fibronectin family and responsible for cell adhesion and protease inhibition, and the receptors for fibroblast growth factor and prokineticin in the autosomal forms.26,27 and 28 Normal GnRH neuron development and migration also depend on receptors that are tyrosine kinases, and abnormalites in these receptors might explain some clinical cases of GnRH deficiency.29 Like olfactory epithelial cells in the nasal cavity, GnRH neurons have cilia.30 The olfactory origin and the structural similarity of GnRH neurons and nasal epithelial cells suggest an evolution from reproduction controlled by pheromones.
 |
Pheromones are airborne chemicals released by one individual that can affect other members of the same species. Odorless compounds obtained from the axillae of women in the late follicular phase of their cycles accelerated the LH surge and shortened the cycles of recipient women, and compounds from the luteal phase had the opposite effects.31 This may be one mechanism by which women who are together much of the time have been reported to exhibit a synchrony in menstrual cycle timing.32,33,34,35 and 36 However, the work on menstrual synchrony has been criticized, emphasizing that methodological problems have led to incorrect conclusions.37
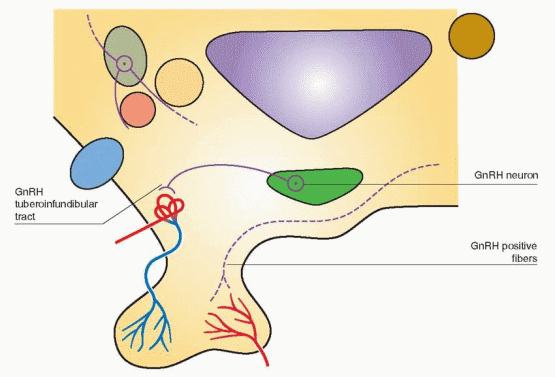
In primates, GnRH cell bodies are primarily located within the medial basal hypothalamus.38,39 and 40 Most of these cell bodies can be seen within the arcuate nucleus where GnRH is synthesized in GnRH neurons. The GnRH neurons exist in a complex network and are connected to each other and to many other neurons. This physical arrangement allows multiple interactions with neurotransmitters, hormones, and growth factors to modulate GnRH release. The delivery of GnRH to the portal circulation is via an axonal pathway, the GnRH tuberoinfundibular tract.
Fibers, identified with immunocytochemical techniques using antibodies to GnRH, can also be visualized in the posterior hypothalamus, descending into the posterior pituitary,
and in the anterior hypothalamic area projecting to sites within the limbic system.38 Using hybridization techniques, messenger RNA for GnRH has been localized to the same sites previously identified by immunoreactivity. However, lesions that interrupt GnRH neurons projecting to regions other than the median eminence do not affect gonadotropin release. Only lesions of the arcuate nucleus in the monkey lead to gonadal atrophy and amenorrhea.41 Therefore, the arcuate nucleus can be viewed with the median eminence as a unit, the key locus within the hypothalamus for GnRH secretion into the portal circulation. The other GnRH neurons may be important for a variety of behavioral responses.
and in the anterior hypothalamic area projecting to sites within the limbic system.38 Using hybridization techniques, messenger RNA for GnRH has been localized to the same sites previously identified by immunoreactivity. However, lesions that interrupt GnRH neurons projecting to regions other than the median eminence do not affect gonadotropin release. Only lesions of the arcuate nucleus in the monkey lead to gonadal atrophy and amenorrhea.41 Therefore, the arcuate nucleus can be viewed with the median eminence as a unit, the key locus within the hypothalamus for GnRH secretion into the portal circulation. The other GnRH neurons may be important for a variety of behavioral responses.
 |
GnRH Secretion
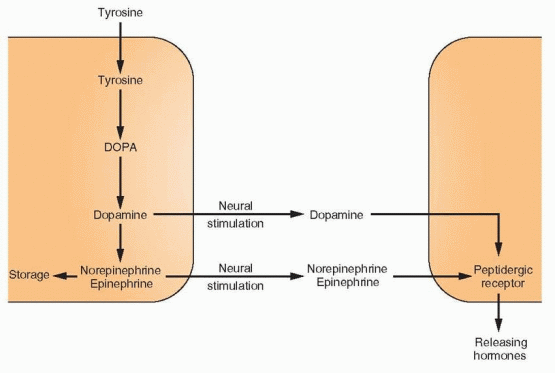
The half-life of GnRH is only 2-4 minutes. Because of this rapid degradation, combined with the enormous dilution on entry into the peripheral circulation, biologically effective amounts of GnRH do not escape the portal system. Therefore, control of the reproductive cycle depends on constant release of GnRH. This function, in turn, depends on the complex and coordinated interrelationships among this releasing hormone, other neurohormones, the pituitary gonadotropins, and the gonadal steroids. The interplay among these substances is governed by feedback effects, both positive stimulatory and negative inhibitory. The long feedback loop refers to the feedback effects of circulating levels of target gland hormones, and this occurs both in the hypothalamus and the pituitary. The short feedback loop indicates a negative feedback of pituitary hormones on their own secretion, presumably via inhibitory effects on releasing hormones in the hypothalamus. Ultrashort feedback refers to inhibition by the releasing hormone on its own synthesis. These signals as well as signals from higher centers in the central nervous system may modify GnRH secretion through an array of neurotransmitters, primarily dopamine, norepinephrine, and endorphin but also serotonin and melatonin. Dopamine and norepinephrine are synthesized in the nerve terminals by decarboxylation of dihydroxyphenylalanine (DOPA), which in turn is synthesized by hydroxylation of tyrosine. Dopamine is the immediate precursor of
norepinephrine and epinephrine, but dopamine itself functions as a key neurotransmitter in the hypothalamus and the pituitary.20
norepinephrine and epinephrine, but dopamine itself functions as a key neurotransmitter in the hypothalamus and the pituitary.20
 |
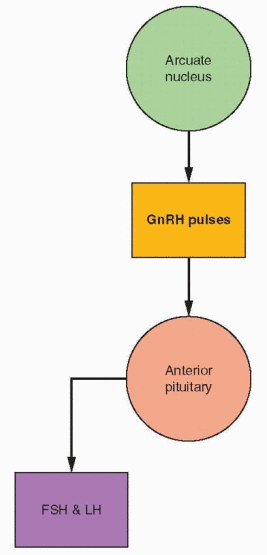
A most useful concept is to view the arcuate nucleus as the central site of action, releasing GnRH into the portal circulation in pulsatile fashion. In a classic series of experiments, it was demonstrated that normal gonadotropin secretion requires pulsatile GnRH
discharge within a critical range in frequency and amplitude.42 Even pituitary hormone gene transcription is sensitive to the pulsatile nature of GnRH release.43
discharge within a critical range in frequency and amplitude.42 Even pituitary hormone gene transcription is sensitive to the pulsatile nature of GnRH release.43
 |
Experimental manipulations have indicated that the critical range of GnRH pulsatile secretion is rather narrow. The administration (to monkeys) of 1 μg GnRH per minute for 6 minutes every hour (one pulse per hour) produces a portal blood concentration about equal to the peak concentration of GnRH in human portal blood, about 2 ng/mL. Increasing the frequency to 2 and 5 pulses per hour extinguishes gonadotropin secretion. A similar decline in gonadotropin secretion is obtained by increasing the dose of GnRH. Decreasing the pulse frequency decreases LH secretion but increases FSH secretion.
Like GnRH, gonadotropins are also secreted in pulsatile fashion, and, indeed, the pulsatile pattern of gonadotropin release reflects the pulsatile GnRH pattern.44,45 GnRH and gonadotropin secretion are always pulsatile in nature, but an augmentation of the pulsatile pattern of gonadotropin secretion occurs just before puberty with nighttime increases in LH. After puberty, enhanced pulsatile secretion is maintained throughout the 24-hour period, but it varies in both amplitude and frequency. In puberty, arcuate activity begins with a low frequency of GnRH release and proceeds through a cycle of acceleration of frequency, characterized by passage from relative inactivity, to nocturnal activation, to the full adult pattern. The progressive changes in FSH and LH reflect this activation of GnRH pulsatile secretion. Ovarian steroid release is also pulsatile, coordinated with LH pulses, the major stimulator of ovarian steroidogenesis.46 In the absence of ovarian regulation, the GnRH pulse frequency is approximately one pulse per hour.47
Timing of GnRH Pulses
The measurement of LH pulses is used as an indication of GnRH pulsatile secretion (the long half-life of FSH precludes its use for this purpose).48 The characteristics of LH pulses (and presumably of GnRH pulses) during the menstrual cycle are as follows:46,49,50
LH Pulse Mean Amplitude: | ||
Early follicular phase | 6.5 IU/L. | |
Midfollicular phase | 5.0 IU/L. | |
Late follicular phase | 7.2 IU/L. | |
Early luteal phase | 15.0 IU/L. | |
Midluteal phase | 12.2 IU/L. | |
Late luteal phase | 8.0 IU/L. | |
LH Pulse Mean Frequency: | ||
Early follicular phase | 90 minutes. | |
Late follicular phase | 60-70 minutes. | |
Early luteal phase | 100 minutes. | |
Late luteal phase | 200 minutes | |
Pulsatile secretion is more frequent but lower in amplitude during the follicular phase compared with the luteal phase. The slowing of GnRH pulse frequencies in the late luteal phase is an important change, favoring FSH synthesis and secretion; therefore, allowing the rise in FSH essential for the next cycle.51 An increase in frequency and amplitude in mid-cycle GnRH pulsatile secretion favors the surge of LH necessary for ovulation and the beginning of the luteal phase.
It should be emphasized that these numbers are not inviolate. There is considerable variability between and within individuals, and a wide normal range exists.52 Despite the
handicap of the long half-life, it has been ascertained that FSH secretion is correlated with LH secretion. The changes in amplitude are relatively small; therefore, increasing and decreasing circulating levels of the gonadotropins are affected mostly by changes in pulse frequency. During the luteal-follicular transition, pulse frequency increases approximately 4.5-fold.50 In rodents, the frequency of GnRH pulses determines which gonadotropin subunit gene will be preferentially expressed.53
handicap of the long half-life, it has been ascertained that FSH secretion is correlated with LH secretion. The changes in amplitude are relatively small; therefore, increasing and decreasing circulating levels of the gonadotropins are affected mostly by changes in pulse frequency. During the luteal-follicular transition, pulse frequency increases approximately 4.5-fold.50 In rodents, the frequency of GnRH pulses determines which gonadotropin subunit gene will be preferentially expressed.53
 |
The anterior pituitary gland and GnRH neurons have an intrinsic pulsatile pattern. Although pulses of significant amplitude are linked to GnRH, small-amplitude pulses of high frequency represent spontaneous secretion from the anterior pituitary (at least as demonstrated in isolated pituitary glands in vitro).54 It is not known whether this has any importance physiologically, and, at the present time, the pituitary secretory pattern is thought to reflect GnRH. The pulsatile secretion of GnRH correlates with episodic GnRH-I gene expression in the hypothalamus.8 A promoter site in the GnRH-I gene is responsible for the pulsatile nature of the secretions, regulated by the usual array of transcription factors.55
Control of GnRH Pulses
Normal menstrual cycles require the maintenance of the pulsatile release of GnRH within a critical range of frequency and amplitude. Pulsatile, rhythmic activity is an intrinsic property of GnRH neurons, and the effect of various hormones and neurotransmitters must be viewed as modulating actions.56
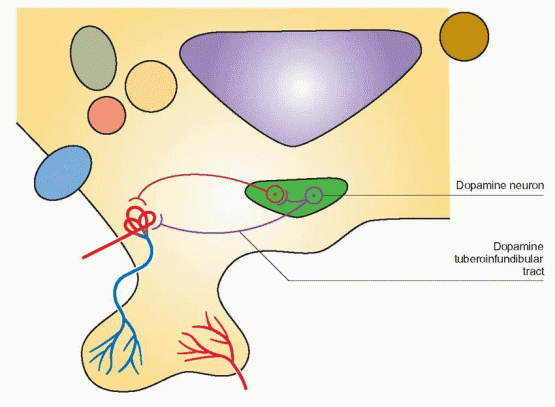
The Dopamine Tract
Cell bodies for dopamine synthesis can be found in the arcuate and periventricular nuclei. The dopamine tuberoinfundibular tract arises within the medial basal hypothalamus and its
short axons terminate in the median eminence; it provides the major dopaminergic effect on the pituitary.
short axons terminate in the median eminence; it provides the major dopaminergic effect on the pituitary.
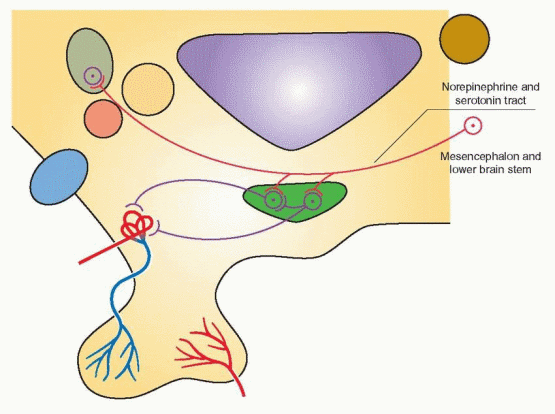 |
The administration of dopamine by intravenous infusion to men and women is associated with a suppression of circulating prolactin and gonadotropin levels.57 Dopamine does not exert a direct effect on gonadotropin secretion by the anterior pituitary; thus, this effect is mediated through GnRH release in the hypothalamus. Dopamine is directly secreted into the portal blood, thus behaving like a neurohormone. Therefore, dopamine may directly suppress arcuate GnRH activity and also be transported via the portal system to directly and specifically suppress pituitary prolactin secretion. The hypothalamic tuberoinfundibular dopamine pathway is not the only dopamine pathway in the CNS, and it is only one of two major dopamine pathways in the hypothalamus. But it is this pathway that directly participates in the regulation of prolactin secretion. In addition, prolactin delivered to the intermediate lobe of the pituitary suppresses melanocyte-stimulating hormone release.
The Norepinephrine Tract
Most of the cell bodies that synthesize norepinephrine are located in the mesencephalon and lower brainstem. These cells also synthesize serotonin. Axons for amine transport ascend into the medial forebrain bundle to terminate in various brain structures including the hypothalamus.
The biogenic catecholamines modulate GnRH pulsatile release.58 Norepinephrine is thought to exert stimulatory effects on GnRH, whereas dopamine and serotonin exert inhibitory effects. For an understanding of clinical problems, it is best to view dopamine as an inhibitor of both GnRH and prolactin. Little is known, however, about the role of serotonin. The probable mode of action of catecholamines is to influence the frequency (and perhaps the amplitude) of GnRH discharge. Thus, pharmacologic or psychologic factors that affect pituitary function probably do so by altering catecholamine synthesis or metabolism and, thus, the pulsatile release of GnRH.
Neuropeptide Y
Neuropeptide Y is an important peptide in the mechanism by which leptin and insulin inform the hypothalamus about the nutritional state of an individual. The secretion and gene expression of neuropeptide Y in hypothalamic neurons are regulated by gonadal steroids.59 Neuropeptide Y stimulates appetite and the pulsatile release of GnRH; in the pituitary it potentiates gonadotropin response to GnRH.60 It, thus, may facilitate pulsatile secretion of GnRH and gonadotropins. In the absence of estrogen, neuropeptide Y inhibits gonadotropin secretion. Because undernutrition is associated with an increase in neuropeptide Y (see Chapter 19) and increased amounts have been measured in cerebrospinal fluid of women with anorexia and bulimia nervosa, neuropeptide Y is viewed as at least one link between nutrition and reproductive function.61,62 The level of the neuroendocrine activity involved in reproduction is sensitive to an individual’s energy state, or more simply, to the availability of sufficient body fuel to support reproduction.
 |
Kisspeptins
Kisspeptins are peptides encoded by the gene Kiss1 that is expressed in the hypothalamus. Kisspeptin and its G-protein receptor, GPR54, are essential for the normal development of puberty.63 This signaling pathway is involved in the activation of GnRH neurons and GnRH secretion.64,65 The kisspeptin neurons contain estrogen and progesterone receptors, and are
involved not only in puberty but in the changes associated with ovulatory cycles, in at least one pathway by which the sex steroids affect GnRH secretion.
involved not only in puberty but in the changes associated with ovulatory cycles, in at least one pathway by which the sex steroids affect GnRH secretion.
Pituitary Gonadotropin Secretion
The gene for the a subunit of the gonadotropins is expressed in both the pituitary and placenta. The b subunit for human chorionic gonadotropin (hCG) is expressed in the placenta but only minimally (and with alterations in structure) in the pituitary, whereas the LH b subunit, as expected, is expressed in the pituitary but not significantly in the placenta.66,67 Studies of gonadotropin gene expression confirm the relationships established by earlier studies. The sex steroids decrease and castration increases the rate of gonadotropin gene transcription as reflected by the levels of specific messenger RNAs. In addition, the sex steroids can act at the membrane level, affecting the interaction of GnRH with its receptor.68
Both LH and FSH are secreted by the same cell, the gonadotrope, localized primarily in the lateral portions of the pituitary gland and responsive to the pulsatile stimulation by GnRH. GnRH is calcium dependent in its mechanism of action and utilizes inositol 1,4,5-triphosphate (IP3) and 1,2-diacylglycerol (1,2-DG) as second messengers to stimulate protein kinase and cyclic AMP activity (Chapter 2).69 These responses require a G protein receptor and are associated with cyclical release of calcium ions from intracellular stores and the opening of cell membrane channels to allow entry of extracellular calcium. Thus, calmodulin, protein kinase, and cyclic AMP are mediators of GnRH action. Gonadotrope gene transcription is mediated by several transcription factors, providing a mechanism by which the various subunits of FSH and LH can be synthesized and secreted by the same cell.70 The rate-limiting step for the secretion of FSH and LH is the synthesis of the beta-subunits of each gonadotropin.
The GnRH type I receptor, a member of the G protein family, is encoded by a gene on chromosome 14q13.1-q21.1.71,72 The location of the type II receptor is uncertain; in the marmoset, it is on chromosome 1q.73 The exact roles of the two GnRHs and GnRH receptors in the human are yet to be determined. GnRH receptors are regulated by many agents, including GnRH itself, inhibin, activin, and the sex steroids.74 The number of GnRH receptors available is significantly regulated by the frequency of GnRH pulses. The signaling pathways include the induction and modification of stimulatory and inhibitory transcription factor proteins. No mutations in the GnRH gene have been described in patients with hypogonadotropic hypogonadism; however, multiple mutations in the GnRH receptor gene have been reported.
Synthesis of gonadotropins takes place on the rough endoplasmic reticulum. The hormones are packaged into secretory granules by the Golgi cisternae of the Golgi apparatus and then stored as secretory granules. Secretion requires migration (activation) of the mature secretory granules to the cell membrane where an alteration in membrane permeability results in extrusion of the secretory granules in response to GnRH. The rate-limiting step in gonadotropin synthesis is the GnRH-dependent availability of the beta subunits.
Binding of GnRH to its receptor in the pituitary activates multiple messengers and responses. The immediate event is a secretory release of gonadotropins, whereas delayed responses prepare for the next secretory release. One of these delayed responses is the self-priming action of GnRH that leads to even greater responses to subsequent GnRH pulses due to a complex series of biochemical and biophysical intracellular events. This self-priming action is important to achieve the large surge in secretion at midcycle; it requires estrogen exposure, and it can be augmented by progesterone. This important action of progesterone depends on estrogen exposure (for an increase in progesterone receptors) and activation of the progesterone receptor by GnRH-stimulated phosphorylation. This latter action is an example of cross-talk between peptide and steroid hormone receptors.
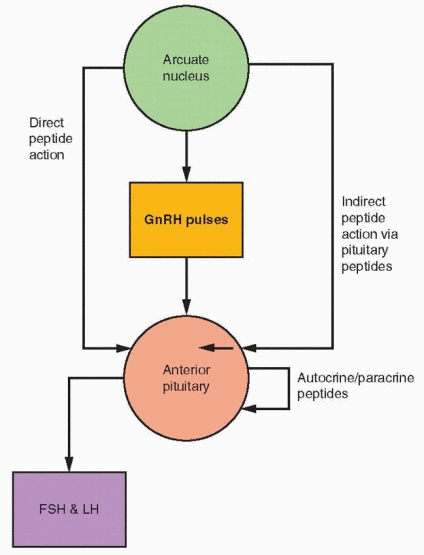 |
Five different types of secretory cells coexist within the anterior pituitary gland: gonadotropes, lactotropes, thyrotropes, somatotropes, and corticotropes. Autocrine and paracrine interactions combine to make anterior pituitary secretion subject to more complicated control than simply reaction to hypothalamic-releasing factors and modulation by feedback signals. Substantial experimental evidence exists to indicate stimulatory and inhibitory influences of various substances on the pituitary secretory cells. Although the GnRH system is a primary mechanism, other hypothalamic peptides can influence GnRH secretion. Peptides can interact with GnRH at the pituitary; peptides can be transported to the pituitary gland where they can directly affect the gonadotropes (e.g., oxytocin, CRH, and neuropeptide Y) or indirectly have an effect on FSH and LH secretion by stimulating the release of active substances within the pituitary (e.g., glalanin, interleukins). Autocrine-paracrine activities involve peptides synthesized by pituitary cells.
The Intrapituitary Autocrine-Paracrine System
Intrapituitary cytokines and growth factors provide an autocrine-paracrine system for regulating pituitary cell development and replication as well as pituitary hormone synthesis and secretion. The pituitary contains the familiar cast of substances encountered in organs throughout the body, including the interleukins, epidermal growth factor, fibroblast growth factors, the insulin-like growth factors, nerve growth factor, activin, inhibin, endothelin, and many others.75,76 and 77 As in most tissues, the interaction among these substances is a complex story, but the activin-inhibin mechanism deserves emphasis.
Activin, Inhibin, and Follistatin
Activin and inhibin are peptide members of the transforming growth factor-β family.78 Inhibin consists of two dissimilar peptides (known as alpha and beta subunits) linked by disulfide bonds. Two forms of inhibin (inhibin-A and inhibin-B) have been purified, each containing an identical alpha subunit and distinct but related beta subunits. Thus, there are three subunits for inhibins: alpha, beta-A, and beta-B. Each subunit is a product of different messenger RNA; therefore, each is derived from its own large precursor molecule.
Inhibin is secreted by granulosa cells, but messenger RNA for the alpha and beta chains has also been found in pituitary gonadotropes.79 Inhibin selectively inhibits FSH but not LH secretion. Indeed, while suppressing FSH synthesis, inhibin may enhance LH activity.80,81 Cells actively synthesizing LH respond to inhibin by increasing GnRH receptor number; FSH dominant cells are suppressed by inhibin.80 Inhibin has little or no effect on growth hormone, ACTH, and prolactin production. The mechanism of FSH inhibition may be secondary to inhibin competing with activin for the activin receptor.
Activin, also derived from granulosa cells, but present as well in the pituitary gonadotropes, contains two subunits that are identical to the beta subunits of inhibins A and B. In addition, activins have been identified with variants of the beta subunits, designated as beta-C, beta-D, and beta-E.82 The activin beta-C and beta-E genes have been demonstrated to be nonessential in mouse knockout models.83 Activin augments the secretion of FSH and inhibits prolactin, ACTH, and growth hormone responses.81,84,85 and 86 Activin increases pituitary response to GnRH by enhancing GnRH receptor formation.74,87 The effects of activin are blocked by inhibin and follistatin.88 The roles for inhibin and activin in regulating the events of the menstrual cycle are discussed in Chapter 6. In contrast to inhibin’s major role in suppressing FSH secretion, activin has a broad range of activities, involving bone, neurons, wound healing, and autocrine-paracrine functions in many organs.
The Forms of Inhibin | ||
Inhibin-A: | Alpha-BetaA | |
Inhibin-B: | Alpha-BetaB | |
The Forms of Activin | ||
Activin-A: | BetaA-BetaA | |
Activin-AB: | BetaA-BetaB | |
Activin-B: | BetaB-BetaB | |
Activin-C: | BetaC-BetaC | |
Activin-AC: | BetaA-BetaC | |
Activin-E: | BetaE-BetaE | |
Follistatin is a peptide secreted by a variety of pituitary cells, including the gonadotropes.89 This peptide has also been called FSH-suppressing protein because of its main action: inhibition of FSH synthesis and secretion and the FSH response to GnRH, by binding to activin and in that fashion decreasing the activity of activin.90,91 Activin stimulates follistatin production, and inhibin prevents this response.
In summary, GnRH stimulates gonadotropin synthesis and secretion, as well as activin, inhibin, and follistatin. Activin enhances and follistatin suppresses GnRH activity. Evidence in vivo and in vitro indicates that gonadotropin response to GnRH requires activin activity, and gonadotropin response can be blocked by follistatin.92,93 and 94 This relationship contributes to the down-regulation of pituitary gonadotropin secretion by
prolonged GnRH stimulation. Increasing GnRH pulsatile frequency first increases FSH production, and, then with high frequency or continuous GnRH stimulation, follistatin production is increased.92 Selective synthesis and secretion of FSH can be explained by the decrease in the inhibiting factors, inhibin and follistatin, allowing activin to enhance the actions of GnRH, involving the transcription factors that promote FSH beta subunit expression.95 LH secretion is primarily regulated by GnRH, without involvement of the inhibin-activin-follistatin system.
prolonged GnRH stimulation. Increasing GnRH pulsatile frequency first increases FSH production, and, then with high frequency or continuous GnRH stimulation, follistatin production is increased.92 Selective synthesis and secretion of FSH can be explained by the decrease in the inhibiting factors, inhibin and follistatin, allowing activin to enhance the actions of GnRH, involving the transcription factors that promote FSH beta subunit expression.95 LH secretion is primarily regulated by GnRH, without involvement of the inhibin-activin-follistatin system.
 |
The Endogenous Opiates
The most fascinating peptide group is the endogenous opioid peptide family.96 β-Lipotropin is a 91 amino acid molecule that was first isolated from the pituitary in 1964. Its function
remained a mystery for more than 10 years until receptors for opioid compounds were identified, and, by virtue of their existence, it was postulated that endogenous opioid compounds must exist and serve important physiologic roles. Endorphin was a word coined to denote morphine-like action and endogenous origin in the brain.
remained a mystery for more than 10 years until receptors for opioid compounds were identified, and, by virtue of their existence, it was postulated that endogenous opioid compounds must exist and serve important physiologic roles. Endorphin was a word coined to denote morphine-like action and endogenous origin in the brain.
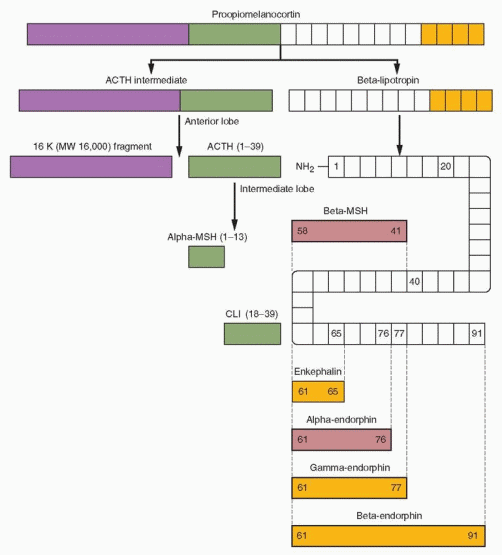
Opiate production is regulated by gene transcription and the synthesis of precursor peptides and at a posttranslational level where the precursors are processed into the various bioactive smaller peptides.97 All opiates derive from one of three precursor peptides.
Proopiomelanocortin (POMC)—the source of endorphins.
Proenkephalin A and B—the source of several enkephalins.
Prodynorphin—yields dynorphins.
POMC was the first precursor peptide to be identified. It is made in the anterior and intermediate lobes of the pituitary; in the hypothalamus and other areas of the brain; in the sympathetic nervous system; and in other tissues including the gonads, the placenta, the gastrointestinal tract, and the lungs. The highest concentration is in the pituitary gland.
Proopiomelanocortin is split into two fragments, an ACTH intermediate fragment and β-lipotropin. β-Lipotropin has no opioid activity but is broken down in a series of steps to β-melanocyte-stimulating hormone (β-MSH), enkephalin; and α-, γ-, and β-endorphins. Melanocyte-stimulating hormone acts in lower animals to stimulate melanin granules within cells, causing darkening of the skin. In humans, there is no known function.
Enkephalin and the α- and γ-endorphins are as active as morphine on a molar basis, whereas β-endorphin is 5-10 times more potent. In the adult pituitary gland, the major products are ACTH and β-lipotropin, with only small amounts of endorphin. Thus, ACTH and β-lipotropin blood levels show similar courses, and they are major secretion products of the anterior pituitary in response to stress. In the intermediate lobe of the pituitary (which is prominent only during fetal life), ACTH is cleaved to CLIP (corticotropin-like intermediate lobe peptide) and β-MSH. In the placenta and adrenal medulla, POMC processing yields a-MSH-like and β-endorphin peptides. β-Endorphin has also been detected in the ovaries and in the testes.
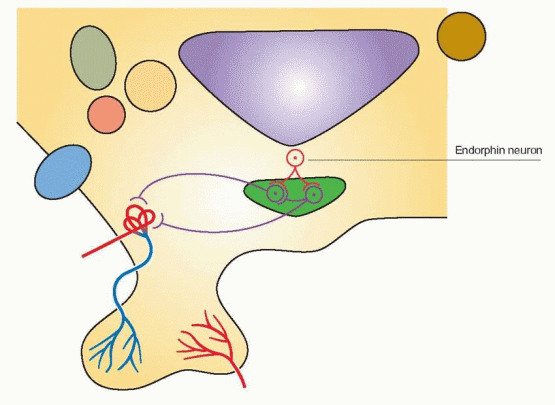
In the brain, the major products are the opiates, with little ACTH. In the hypothalamus the major products are β-endorphin and a-MSH in the region of the arcuate nucleus and the ventromedial nucleus. The pituitary opiate system is a system for secretion into the circulation whereas the hypothalamic opiate system allows for distribution via axons to regulate other brain regions and the pituitary gland.
β-Endorphin is appropriately considered a neurotransmitter, a neurohormone, and a neuromodulator. β-Endorphin influences a variety of hypothalamic functions, including regulation of reproduction, temperature, and cardiovascular and respiratory function, as well as extrahypothalamic functions such as pain perception and mood. POMC gene expression in the anterior pituitary is controlled mainly by corticotropin-releasing hormone and influenced by the feedback effects of glucocorticoids. In the hypothalamus, regulation of POMC gene expression is via the sex steroids.98 In the absence of sex steroids, little, if any, secretion occurs.
Proenkephalin A is produced in the adrenal medulla, the brain, the posterior pituitary, the spinal cord, and the gastrointestinal tract. It yields several enkephalins: methionineenkephalin, leucine-enkephalin, and other variants. The enkephalins are the most widely distributed endogenous opioid peptides in the brain and are probably mainly involved
as inhibitory neurotransmitters in the modulation of the autonomic nervous system. Prodynorphin, found in the brain (concentrated in the hypothalamus) and the gastrointestinal tract, yields dynorphin, an opioid peptide with high analgesic potency and behavioral effects, as well as α-neoendorphin, β-neoendorphin, and leumorphin. The last 13 amino acids of leumorphin constitute another opioid peptide, rimorphin. The prodynorphin products probably function in a fashion similar to endorphin.
as inhibitory neurotransmitters in the modulation of the autonomic nervous system. Prodynorphin, found in the brain (concentrated in the hypothalamus) and the gastrointestinal tract, yields dynorphin, an opioid peptide with high analgesic potency and behavioral effects, as well as α-neoendorphin, β-neoendorphin, and leumorphin. The last 13 amino acids of leumorphin constitute another opioid peptide, rimorphin. The prodynorphin products probably function in a fashion similar to endorphin.
 |
It is simpler to say that there are three classes of opiates: enkephalin, endorphin, and dynorphin.
Opioid peptides are able to act through different receptors, although specific opiates bind predominantly to one of the various receptor types. Naloxone, used in most human studies, does not bind exclusively to any one receptor type, and, thus, results with this antagonist are not totally specific. Localization of opioid receptors explains many of the pharmacologic actions of the opiates. Opioid receptors are found in the nerve endings of sensory neurons, in the limbic system (site of euphoric emotions), in brainstem centers for reflexes such as respiration, and widely distributed in the brain and the spinal cord.
Opioid Peptides and the Menstrual Cycle
The opioid tone is an important part of menstrual function and cyclicity.99 Although estradiol alone increases endorphin secretion, the highest levels of endorphin occur with sequential therapy of both estradiol and progesterone (in ovariectomized monkeys). Endogenous endorphin levels, therefore, increase throughout the cycle from nadir levels during menses to highest levels during the luteal phase. Normal cyclicity, thus, requires sequential periods of high (luteal phase) and low (during menses) hypothalamic opioid activity.
A reduction in LH pulse frequency is linked to increased endorphin release.100 Naloxone increases both the frequency and the amplitude of LH pulses. Thus, the endogenous opiates inhibit gonadotropin secretion by suppressing the hypothalamic release of GnRH. Opiates have no effect on the pituitary response to GnRH. The gonadal steroids modify endogenous opioid activity, and the negative feedback of steroids on gonadotropins appears to be mediated by endogenous opiates. Because the fluctuating levels of endogenous opiates in the menstrual cycle are related to the changing levels of estradiol and progesterone, it is attractive to speculate that the sex steroids directly stimulate endogenous opioid receptor activity. There is an absence of opioid effect on postmenopausal and oophorectomized levels of gonadotropins, and the response to opiates is restored with the administration of estrogen, progesterone, or both.101 Both estrogen and progesterone alone increase endogenous opiates, but estrogen enhances the action of progesterone, which could explain the maximal suppression of GnRH and gonadotropin pulse frequency during the luteal phase.102,103 In pubertal boys and girls, however, naloxone could not prevent the suppression of LH by estradiol administration, indicating that in this circumstance estradiol may directly inhibit GnRH secretion.104,105 Nevertheless, the evidence overall indicates that endogenous opiates exert an inhibiting influence over GnRH secretion.106 The negative feedback of progesterone on GnRH secretion (the major mechanism for the inhibition of ovulation associated with progestin contraception) is definitely partly mediated by endogenous opiates but also by other neural mechanisms not yet determined.107
The inhibiting tone of endogenous opiates is reduced at the time of the ovulatory surge, allowing a release from suppression.108 This is probably a response to estrogen, specifically an estrogen-induced decrease in opioid receptor binding and opioid release.109,110
Experiments with naloxone administration suggest that the suppression of gonadotropins during pregnancy and the recovery during the postpartum period reflect steroid-induced opioid inhibition, followed by a release from central opioid suppression.
The principal endogenous opiates affecting GnRH release are β-endorphin and dynorphin, and it is probable that the major effect is modulation of the catecholamine pathway, principally norepinephrine. The action does not involve dopamine receptors, acetylcholine receptors, or alpha-adrenergic receptors. On the other hand, endorphin may affect GnRH release directly, without the involvement of any intermediary neuroamine.
Because α-MSH counteracts the effects of β-endorphin, posttranslational processing of POMC can affect hypothalamic-pituitary function by altering the amounts of α-MSH and β-endorphin.111 This introduces another potential site for neuroendocrine regulation of reproductive function. Gonadal hormones likely have multiple sites for feedback signals.
Clinical Implications
A change in opioid inhibitory tone is not important in the changes of puberty because the responsiveness to naloxone does not develop until after puberty. A change in opioid tone does seem to mediate the hypogonadotropic state seen with elevated prolactin levels, exercise, and other conditions of hypothalamic amenorrhea, whereas endogenous opioid inhibition does not seem to play a causal role in delayed puberty or hereditary problems such as Kallmann’s syndrome.112,113 Treatment of patients with hypothalamic amenorrhea (suppressed GnRH pulsatile secretion) with a drug (naltrexone) that blocks opioid receptors restores normal function (ovulation and pregnancy).114 Thus, the reduced GnRH secretion associated with hypothalamic amenorrhea is mediated by an increase in endogenous opioid inhibitory tone.
Experimental evidence indicates that corticotropin-releasing hormone (CRH) inhibits hypothalamic GnRH secretion, both directly and by augmenting endogenous opioid secretion. Women with hypothalamic amenorrhea demonstrate hypercortisolism, suggesting that this is the pathway by which stress interrupts reproductive function.115 Mathematical analysis of the associations among FSH, LH, β-endorphin and cortisol pulses support the existence of significant functional coupling between the neuroregulatory systems that control the gonadal and adrenal axes.116 The CRH gene contains two segments that are similar to estrogen response elements, allowing estrogen enhancement of CRH activity, perhaps explaining the greater vulnerability of the reproductive axis to stress in females.117 Besides the CRH-induced inhibition of GnRH release, the increase in cortisol generated by CRH stimulation of pituitary ACTH secretion also contributes to the suppression of reproduction; cortisol directly inhibits pituitary responsiveness to GnRH.118
Cumming concluded that most studies indicate an exercise-induced increase in endogenous opiates, but a significant impact on mood remains to be substantiated.119 He noted that runners’ high is more common in California than in Canada (euphoria is hard to come by when running in below freezing temperatures!).
Administration of morphine, enkephalin analogs, and β-endorphin causes release of prolactin. The effect is mediated by inhibition of dopamine secretion in the tuberoinfundibular neurons in the median eminence. Most studies have reported no effect of naloxone on basal, stress-induced, or pregnant levels of prolactin nor on secretion by prolactinomas. Thus, a physiologic role for endogenous opioid regulation of prolactin does not appear to exist in men and women. However, suppression of GnRH secretion associated with hyperprolactinemia does appear to be mediated by endogenous opiates.120
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


