Neuroimmunology
Stephen C. Marini
Students and clinicians of chiropractic and complementary medicine are often called upon to evaluate and care for patients with disorders that are based upon pathogenic mechanisms involving the neurologic and immunologic systems. These mechanisms involve an understanding of the field of neuroimmunology, a growing branch of biomedical science that encompasses all aspects of the interactions between the immune system and the nervous system. It deals with, among other things, the physiological functioning of the cells and chemical mediators of the neuroimmune system in states of both health and disease and the malfunctions of the system that are seen in patients who present with a variety of disorders including allergies and hypersensitivities, chronic infections, autoimmune diseases, immune deficiencies, and cancer. This chapter will describe the physical, molecular, and physiological characteristics of the components of the neuroimmune system together with an integrative view of the major components of the immune system and the factors contributing to the immune imbalance underlying these disorders and will present a suggested algorithm for the clinical recognition and restoration of immune balance.
Scientific evidence from the fields of neuroendocrinology, neuroimmunology, gastroenterology, and nutrition has revealed that our autonomic, endocrine, and immune systems are not independent and autonomous but, rather, engage in an interactive and interdependent dialogue with one another. Research in psychoneuroimmunology (PNI) has expanded our understanding of the mechanisms of health and illness by establishing the roles of the perceptual, cortical, and limbic systems (limbic system is also called the emotional center of the brain), and stress in immunostasis or immune balance. These studies likewise stress the critical role of the immune system as the central initiating focus to initiate and modulate neuroendocrine and autonomic change. Contained with this complex network of multisystem communication and interaction are a myriad of epigenetic factors which influence the genetic expression of phenotypic disorders which begin in-utero and continue throughout the lifespan of the individual to senescence with the ultimate aim of developing, balancing, and refining the immune system (1,2).
Psychological factors are likewise important in affecting the clinical expressions of the neuroimmunologic system(s). Observations linking psychological disorders to immune imbalance catalyzed Solomon to coin the term Psychoimmunology in 1964 (3). A note appearing in the classic 1971 Bellanti Immunology text linked immune reactions to emotional changes (4). The concept was further refined by the term psychoneuroimmunology (PNI) and reached textbook status with the superlative work of Ader, Cohen, and Felten in 1991 who summarized the research illuminating cellular receptors on immune cells which control signaling pathways within the immune system as well as the neurochemical mediators which perform similar functions in the neurologic system (5). An impediment to recognizing and incorporating the results of the PNI research into medical and public health practice derived, in part, from a reductionistic paradigm which accepted the immune system as an independent and autonomously functioning bodily system (6). The reductionistic or mechanistic paradigm fails to acknowledge the mind, energy, and information as entities in health, healing, and disease prevention. Holistic paradigms embrace the coherent nature of immune functions interacting with all body systems along with the role of the mind, energy, and information on all body systems (6,7,8).
THE COMPONENTS OF THE IMMUNE SYSTEM: THE INNATE AND ADAPTIVE IMMUNE SYSTEMS
The components of the immune system which function to maintain balance between the internal and external environments are grouped under two major compartments,
the innate and acquired immunity system (7,9). Innate or natural immunity include the physical barriers such as skin, mucous membranes, hair, and cilia; chemical barriers such as sweat, tears, saliva, plasma, and stomach acid. The physical and chemical barriers are also considered to be part of our body’s first line of defense. The second line of defense within innate immunity encompasses the cells and cell products that are involved in inflammation, the body’s reaction to foreign stimuli. Inflammation involves the coordinated interaction of granulocytes, macrophages, dendritic cells, mast cells, and natural killer (NK) cells which, together with the soluble factors such as the complement system and antibody, are engaged in phagocytosis, degranulation, antigen processing, and intercellular communication. Innate immunity protects the body against infectious organisms and is constitutively hard wired and is always active, reacts quickly, and has no memory. The role of energy fields and unified fields enveloping the physical body and their participation in the body’s defenses has yet to be defined.
the innate and acquired immunity system (7,9). Innate or natural immunity include the physical barriers such as skin, mucous membranes, hair, and cilia; chemical barriers such as sweat, tears, saliva, plasma, and stomach acid. The physical and chemical barriers are also considered to be part of our body’s first line of defense. The second line of defense within innate immunity encompasses the cells and cell products that are involved in inflammation, the body’s reaction to foreign stimuli. Inflammation involves the coordinated interaction of granulocytes, macrophages, dendritic cells, mast cells, and natural killer (NK) cells which, together with the soluble factors such as the complement system and antibody, are engaged in phagocytosis, degranulation, antigen processing, and intercellular communication. Innate immunity protects the body against infectious organisms and is constitutively hard wired and is always active, reacts quickly, and has no memory. The role of energy fields and unified fields enveloping the physical body and their participation in the body’s defenses has yet to be defined.
The adaptive or acquired immune system occurs after exposure to an immunogen, involves lymphocytes, and demonstrates exquisite specificity and memory. An immunogen is usually a foreign substance such as an infectious organism or toxin capable of stimulating a lymphocytic response specifically directed to that substance. The resulting response can be the production of specific antibody or activation of a cell-mediated reaction to the antigen. When the acquired immune system encounters an antigen to which it has already been exposed or sensitized to, it responds more rapidly than it did to the primary interaction. The secondary or challenge response occurs quickly with a greater magnitude for antibody and cell-mediated responses. Subsequent exposures to the same specific antigen will generate the same magnitude, secondary-type response. Memory responses to specific antigens can be permanent or temporary depending on the nature of the immunogen, mechanism and quantity of exposure, and the type of acquired immune reaction chosen.
THE LYMPHOCYTES OF THE ADAPTIVE IMMUNE SYSTEM: T AND B CELLS
Two universes of lymphocytes are involved in the adaptive immune system responses. These include the T or thymus-derived lymphocytes and the B or bone marrow (bursa equivalent) lymphocytes (10,11). In the human, the thymus gland develops from the third and fourth pharyngeal pouches during embryogenesis and serves to mature precursor lymphocytes into functional T cells through a highly selective process of clonal expansion and deletion of unwanted T cell precursors which otherwise would be autoreactive against one’s own tissues and lead to autoimmune disease. Mature T cells which emerge from the thymus are capable of recognizing and protecting self-antigens known as the HLA (human leukocyte antigens) which are controlled and produced by the genes of the major histocompatibility complex (MHC). Once released from the primary thymic tissue, T cells are free to circulate in the peripheral blood and take up residence in reserved areas of the spleen and lymph nodes. Populations of mature T cells are further differentiated by the presence of unique cell surface markers. CD4 subsets of T cells are classified as helper T cells and are further distinguished as CD4-Th1 and CD4-Th2. CD8 T cells are called cytotoxic lymphocytes capable of killing cells infected by viruses or as directed by T helper cells. Other T cell subtypes have recently been discovered which include Treg cells (Th3 and Tr1) and T17 cells which are responsible for regulation and suppression of all immune reactions and inflammatory reactions, respectively. The Th1 and Th2 cells orchestrate specific types and scopes of reactions including interacting with the nervous, endocrine, and other systems as well as maintaining specific memory for further responses.
THE ROLE OF TH1 CELLS
T helper type 1 (Th1) cells direct responses designated as CMI (cell-mediated immunity) (7,8,9,10,11). The Th1 cells help macrophages to kill the bacteria they ingest through phagocytosis, help activate cytotoxic lymphocytes to kill virally infected cells, and regulate Th2 responses. The T cells communicate with lymphocytes, monocytes, macrophages, neurons, endocrine cells, and other tissue cells by means of soluble cytokine proteins. Cytokines from cells of the immune system and other tissues influence the activity of other cells by binding on cell membrane receptors thereby regulating and mediating immune, nervous, endocrine, and inflammatory responses. Examples of Th1 cytokines include interleukin 2 (IL-2), gamma interferon, IL-3, IL-12, IL-18, and tumor necrosis factor beta (TNFbeta). Additionally, gamma interferon serves partly in suppressing Th2 function. Th1 responses are necessary for defense against viral intracellular infections, gram-negative bacterial, tuberculosis, fungal, and parasitic infections as well as provide surveillance against cancer and foreign tissue. Deficiencies in the Th1 cell compartment render individuals susceptible to opportunistic infections and malignancy. The coordinated efforts of the Th1 cells, their communication chemicals, and the tissues regulated generate externalization type responses. For example, in defense against the influenza virus the Th1 cells orchestra the production of specific flu-neutralizing antibodies and stimulate macrophage elimination and cytotoxic cell destruction
of cells already infected with the virus. In addition, the Th1 cells assist in regulating fever, secretions, coughing, and sneezing responses to externalize and hasten the recovery from infection. Th1 responses to viral infections such as measles, mumps, rubella (MMR), chicken pox, and so on confer permanent, lifelong immunity. Crucial to the conversion of uncommitted T cells into Th1 cells is the involvement of the dendritic cell (12). Antigen processing and cytokine production by dendritic cells serve as a natural adjuvant or stimulus for cell-mediated immune reactions. Current vaccine strategies eliminate dendritic cell involvement thereby precluding natural Th1 responses to many vaccine types, with Th2 humoral immune responses induced instead (13,14).
of cells already infected with the virus. In addition, the Th1 cells assist in regulating fever, secretions, coughing, and sneezing responses to externalize and hasten the recovery from infection. Th1 responses to viral infections such as measles, mumps, rubella (MMR), chicken pox, and so on confer permanent, lifelong immunity. Crucial to the conversion of uncommitted T cells into Th1 cells is the involvement of the dendritic cell (12). Antigen processing and cytokine production by dendritic cells serve as a natural adjuvant or stimulus for cell-mediated immune reactions. Current vaccine strategies eliminate dendritic cell involvement thereby precluding natural Th1 responses to many vaccine types, with Th2 humoral immune responses induced instead (13,14).
THE ROLE OF TH2 CELLS: WITH B CELLS MAKE ANTIBODY
Th2 lymphocytes direct humoral immune responses in which they induce B lymphocytes to mature into antibody-producing plasma cells (7,8,9,10,11). Humoral responses occur in the serum and other extracellular body compartments in which antibodies, produced specifically to an immunogen stimulus, bind with the antigen, forming an immune complex. Immune complexes initiate a reaction cascade involving the complement system by allowing the body to clear the complex from the circulation along with the liver and spleen. Th2-mediated responses are needed in defense of gram-positive bacterial infections, toxins from bacterial infection and the environment, and the extracellular phase of viral infections. Th2 cells direct the production of IgE-type antibodies which are responsible for allergic, immediate-type hypersensitivity reactions resulting in the symptoms of allergies and asthma. Cytokines produced by the Th2 cells include IL-4, IL-5, IL-6, IL-10, and IL-13. These cytokines dialogue with other immune cells as well as the neuroendocrine system and other tissues. IL-10 functions partly in the suppression of Th-1 cell function and Th-1 cytokine production. Th2 responses are characterized by internalization-type responses in which antigen-antibody complexes are filtered, phagocytized, and intracellularly digested. Conventional vaccines serve to elicit Th2 mediated, high-titer antibody responses which are quick reacting and are of a temporary nature requiring frequent booster exposures of the specific antigen (15,16,17,18,19).
NK lymphocytes (NK cells) are different from T or B cells, contain cytoplasmic granules, and have the capability of killing virally infected cells and those that have undergone malignant transformation. Unlike their T cell counterparts, they do not have the ability of recognizing the specificity of the virus infecting a cell.
THE ROLE OF B CELLS
B lymphocytes (B cells) acquired their name originally from the B cell maturation organ in the chicken called the Bursa of Fabricius (9,10,11). Mammalian systems make use of the bone marrow for the maturation of pluripotent stem cells into functional B cells. These cells are a vital component of adaptive humoral immunity possessing the principal function of antibody production. The B cell maturation process involves several stages of genetic rearrangements within two major components of the immunoglobin molecule, the antibody heavy (H) and light (L) chain loci. As with its T cell counterparts, B cells recognize self-antigens during the maturation process. If the maturing B cell fails to self-recognize, cell death or apoptosis leading to clonal deletion occurs. Each mature B cell displays a unique receptor protein (BCR) on its cell membrane which is capable of binding to one particular antigen. The protein is a membranebound immunoglobulin (Ig) which serves to distinguish the B cell from all other lymphocytes as well as being the main antibody protein produced upon B cell activation. Upon exposure to antigen and T helper cell cytokine signaling, the B cell differentiates into antibody factories (plasma cells) and memory B cells. The B cells recognize their specific antigen in its native form in the blood or lymph using their BCR. In contrast, T cells recognize antigens on their T cell receptor (TCR) in a processed form presented by an antigen-presenting cell. Mature B cells possess reserved regions in the spleen and lymph nodes called germinal centers.
THE PRODUCTS OF B CELLS: THE IMMUNOGLOGULINS
Antibodies produced by activated B cells or plasma cells belong to five distinct Ig classes (9,10,11). IgM, IgG, IgA, IgE, and IgD differ in the constant regions of their polypeptide chains and purpose in body defense strategies. The variable portions of their heavy and light chains possess the stereospecificity necessary to specifically interact with antigen. IgM developed first among animal humoral defense systems and it is the main Ig class of primary or sensitization immune responses. IgM is a large pentameric protein not capable of passing through the placenta or into the breast milk. The fetus is capable of producing this class of antibodies in response to in-utero infections. The IgM antibodies agglutinate cells through cross-linking, stimulate macrophage phagocytosis, and activate the complement system for cell destruction and phagocytosis. Antibodies of the IgG class are the major antibodies of defense and are produced at homeostatic high titers in response to a secondary or challenge infection. Subsequent exposures
to the same antigen will stimulate the same secondary titer of IgG antibody. This class antibody does traverse the placental barrier and can be found in breast milk. Levels of IgG antibody rise in half the time consequential to a secondary exposure to the same antigen. Booster vaccinations function to restore IgG antibody levels to secondary response levels. Primary and booster vaccinations elicit high-titer antibody responses that are mainly Th2 directed. IgG2 antibodies are a subset antibody class induced by Th1 reactions and typify natural immune responses to microorganisms. The goal of vaccine strategies is to stimulate and maintain high titers of antigen-specific IgG antibodies against targeted microorganisms throughout the life of an individual through repeated booster vaccinations. The IgG antibodies activate the complement system and stimulate macrophage ingestion of antigenic particles by coating them with antibodies. The IgA class antibodies are found in body secretions including colostrum and breast milk and provide defense in the mucous membrane areas of the body especially the gastrointestinal tract of the nursing infant. Contemporary injection-type vaccine strategies bypass the body’s natural lines of defense and fail to provoke mucous membrane IgA production and the protection afforded by this secretory antibody (20,21,22). IgE class antibodies circulate in the peripheral blood and attach to basophils and mast cells. Upon interaction with a specific antigen, IgE antibodies catalyze the degranulation of these cells resulting in histamine reactions and anaphylaxis. IgE antibodies reach high levels in immediate hypersensitivity or allergic conditions. Production of IgE antibodies is a Th2-mediated process. IgE antibody activity is essential in body defenses against parasitic infections. The production of IgE antibodies to a host of environmental antigens characterizes individuals who possess multiple allergies, asthma, and so on and reflects an overactive and aggressive Th2 arm of immune responsiveness as well as a dysfunctional regulatory process. The IgD class does not serve in defense activities and is important in B cell structure.
to the same antigen will stimulate the same secondary titer of IgG antibody. This class antibody does traverse the placental barrier and can be found in breast milk. Levels of IgG antibody rise in half the time consequential to a secondary exposure to the same antigen. Booster vaccinations function to restore IgG antibody levels to secondary response levels. Primary and booster vaccinations elicit high-titer antibody responses that are mainly Th2 directed. IgG2 antibodies are a subset antibody class induced by Th1 reactions and typify natural immune responses to microorganisms. The goal of vaccine strategies is to stimulate and maintain high titers of antigen-specific IgG antibodies against targeted microorganisms throughout the life of an individual through repeated booster vaccinations. The IgG antibodies activate the complement system and stimulate macrophage ingestion of antigenic particles by coating them with antibodies. The IgA class antibodies are found in body secretions including colostrum and breast milk and provide defense in the mucous membrane areas of the body especially the gastrointestinal tract of the nursing infant. Contemporary injection-type vaccine strategies bypass the body’s natural lines of defense and fail to provoke mucous membrane IgA production and the protection afforded by this secretory antibody (20,21,22). IgE class antibodies circulate in the peripheral blood and attach to basophils and mast cells. Upon interaction with a specific antigen, IgE antibodies catalyze the degranulation of these cells resulting in histamine reactions and anaphylaxis. IgE antibodies reach high levels in immediate hypersensitivity or allergic conditions. Production of IgE antibodies is a Th2-mediated process. IgE antibody activity is essential in body defenses against parasitic infections. The production of IgE antibodies to a host of environmental antigens characterizes individuals who possess multiple allergies, asthma, and so on and reflects an overactive and aggressive Th2 arm of immune responsiveness as well as a dysfunctional regulatory process. The IgD class does not serve in defense activities and is important in B cell structure.
TWO TYPES OF ANTIGENS: T-DEPENDENT AND T-INDEPENDENT ANTIGENS
Antigens are grouped in two ways based on B cell interactions: (a) those antigens which require T cell help to stimulate B cells, the T-dependent antigens; and (b) those antigens which can interact directly with B cells stimulating antibody production and do not require T cell help, that is, the T-independent antigens (9,10,11). Most antigens are proteins and are dependent on T cell help for maximal antibody production (23). Cytokines from activated T cells stimulate B cell differentiation into plasma cells as well as the Ig class switch to IgG, IgA, IgE, and memory cell formation. T-independent antigens, consist mainly of polysaccharides and are capable of delivering all the necessary signals to the B cells for stimulating plasma cells and producing antibodies.
THE ROLE OF THE NEUROLOGIC SYSTEM IN THE NEUROIMMUNE NETWORK
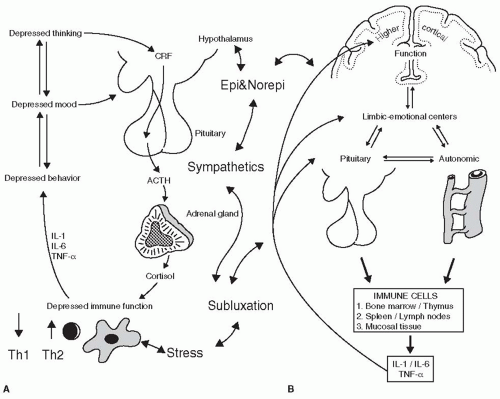
The autonomic nervous system possesses neuroimmunemodulating capabilities incorporating the parasympathetic, sympathetic, non-adrenergic, and non-cholinergic pathways (6,7,8). These pathways have been found to innervate lymphoid tissue, bone marrow, thymus, gastrointestinal, and respiratory tract lymphoid tissues. All aspects of inflammation and immune responsiveness are fine-tuned by these pathways. Cytokines participating in this dialogue include excitatory acetylcholine, neuropeptide T, substance P, inhibitory epinephrine, nitric oxide, and vasoactive intestinal polypeptide (7). Neurologic-based cytokines can change during the course of an immune response resulting in the up- or downregulation of other neurologic responses. Major neuroendocrine influences on immune function result from TCR interaction with Gh, TRH, TSH, HCG, arginine vasopressin, GdRH, androgens, prolactin, alpha, beta, and gamma endorphin, enkephalins, and ACTH. Immunoneurologic signals include IL-1, 6, TNF, and leukosteroid stress peptides. These cytokines promote fever, malaise, fatigue, diminished feeding, and drinking responses, and reduce social interactions. Cortical involvement in neuroimmunomodulation integrates three cortical centers of autonomic control (6). Firstly, the insular cortex integrates emotional and autonomic responses through connections with the lateral hypothalamus, central amygdalar nucleus, parabrachial nucleus, parasympathetic preganglionic nucleus in the medulla, nucleus of the tractus solitaries, thalamus, and the reticular activating system. Secondly, the medial prefrontal or infralimbic cortex responds to emotions and stress possessing extensive hippocampal and amygdalar inputs and outputs to the intermediolateral cell column. Thirdly, the sensorimotor cortex contains various central nuclei mediating pressor responses to different behavioral stimuli and emotional states. Animal studies conclude that left cortical ablation results in immune suppression with immune enhancement following right cortical removal. Within the limbic system, amygdalar ablation generates immune enhancement and immune suppression resulting from hippocampal removal. Immune enhancement also follows raphe nucleus and locus ceruleus ablation. Studies also point to immunosuppression resulting from
persistent high-ACTH levels uncoupling the corticoamygdalar connection. Left cortical stimulation via generating lists, numbers, order, and controlling activities also improves immune function.
persistent high-ACTH levels uncoupling the corticoamygdalar connection. Left cortical stimulation via generating lists, numbers, order, and controlling activities also improves immune function.
Additionally, stress, anger, and despair dispositions activate three neuroendocrine pathways. Central autonomic nuclei, that is, the central amygdalar nucleus for norepinephrine-mediated fight responses and the basal amydalar nucleus for epinephrine-mediated flight responses. The sympathetic adreno-medullary axis is activated by central autonomic responses resulting in increased epinephrine. Hypothalamic-pituitary-adrenal axis (HPAC) expression correlates with depression, defeat, and despair based consciousness patterns. The three neuroendocrine pathways serve as immunosuppressives resulting in the production of lymphoid cytokines that further induce autonomic depression. The significance of excess sympathetic tone and the role of the spinal cord as an organizer of disease has been reported by Korr (24). Correlation of segmental spinal lesions or vertebral subluxation with sympathetic stimulus suggests a role of spinal lesions in neuroendocrine and immune functions (25,26,27,28,29). Depressed psychological patterns result in depressed immunologic functional patterns; depressed immunologic patterns beget depressed psychological patterns. Ader’s work validates the ability to classically condition the immune system by means similar to classic neurologic and psychological conditioning (5).
THE ROLE OF EMOTIONS IN IMMUNE FUNCTION: PSYCHONEUROIMMUNOLOGY
Recent well-designed studies reviewed by Vedhara (8) and Watkins (6) have applied the advances in PNI to health care issues including chronic viral infections, AIDS, cancer, and chronic degenerative diseases.
Evidence from epidemiological studies reveals psychosocial factors affecting T cell and cytokine levels, neurohormone levels, and genetic changes which factor into the onset and pathogenesis of disease states (30,31). Interferences as well as patterns of interference within the PNI framework modify resistance to infection and increase the relative pathogenicity of microorganisms (32,33,34).
Stress, depression, and insomnia are known to alter the circulating levels of neuroendocrine hormones and cytokines, which have the potential to alter the immune function (35,36,37,38,39,40). Such changes in immune competence relate to the development and progression of cancer (41). The Th1 response is thought to be the predominant response required for immune surveillance against cancer as well as the anticancer response (42). Conversely, Th2 cells have a cytokine repertoire conducive to a humoral immune response that is not anticancer effective and are characterized by specific antibody formation. A shift in the Th1-Th2 balance results from psychoneuroendocrine stress resulting in a Th2 prevalent response (43,44,45). The anticancer role of the dendritic cell has recently attracted attention especially regarding their T-cell-activating functions and dependence on the cytokine environment (46). In addition to direct effects on lymphocytes and cytokine levels, neuroendocrine hormones influence the immune system at the genetic level (47,48,49,50,51,52). Hormone regulation of interferon regulatory factor (53) and suppressor of cytokine signaling protein expression (54) as well as the ability of hypothalamic-pituitary-adrenal hormones promoting breast cancer oncogene expression (34) has been documented.
A clearer picture of the interactions among stressful experiences, depression, imbalanced T cell levels, abnormal cytokine patterns, aggressive inflammation, central nervous system (CNS) and neuroendocrine reactions, and epigenetic changes has arisen from consistent evidence within the fields of infectious disease and vaccination neuroimmunology. Miller and Cohen (55) have reviewed the literature linking PNI imbalances to acute, chronic, and latent infectious diseases. Evidence is strongest for acute infectious diseases such as influenza and colds, which are the most common forms of infectious disease and contributors to worldwide morbidity and mortality. The reviewers assert that stressful experiences show a dose-response relationship with illness vulnerability such that cold and influenza risk increase linearly with both the duration of a stressor as well as its perceived severity. Ongoing chronic stressors heighten one’s susceptibility to developing clinical illness two- to threefold. Similar results suggest that long-term stressors increase the vulnerability to clinical outbreaks of latent infections such as the herpes virus family (55,56). Temporary stressors do not seem to have the same impact on vulnerability as patterns of PNI interference. The Th1-cell-mediated arm of immunity is known to be the required and appropriate defense response against intracellular infections including viruses as well as gram-negative bacteria, tuberculosis, and fungi. Incorporated into the Th1 response to viruses is the capability to generate antibodies for virus neutralization as well as an efficient mechanism to identify and eliminate cells containing viruses. Although the Th2 humoral response is effective in generating specific virus-neutralizing antibody, it is not adept at eliminating virally infected cells (44,57). In the case of latent viral infections, the rise in specific antibody titers correlates with increased stressors and increased viral replication (55). The presence of antibody in these cases is not a marker of immunity but of chronic ongoing infection (18). Research on chronic HIV suggests that as the virus progresses, the cytokine profile shifts
from the production of predominately Th1 cytokines to the production of predominately Th2 cytokines (58,59). Catecholamines and glucocorticoids fuel the switch from Th1 to Th2 cytokine production pattern resulting in decrements in lymphocyte numbers, cytotoxic T cell function, NK cell count, and viral surveillance. HIV viremia results from this sequence followed by an increased risk of HIV morbidity and mortality. The importance of a Th1 to Th2 cytokine shift demonstrated in HIV infections stems from the ability of Th1 cytokines to decrease the vulnerability of lymphocytes to apoptosis and inhibiting HIV replication, while Th2 cytokines increase the susceptibility to apoptosis and stimulate HIV replication (58,59). Research over the past two decades has elucidated a reciprocal relationship between the mind and body especially within a framework of anti-microbial immunity. Psychosocial factors including stress, depression, bereavement, medications, and so on create a pattern of interference on neuroendocrine function and outcomes of immunity. The switch from Th1 to Th2 is followed by increasing antibody titers to latent viral infections resulting in poorer viral immunosurveillance. Distressed individuals exhibit heightened sympathetic and HPAC activity further compromising antimicrobial immunity (60,61). Norepinephrine is associated with an increase in HIV replication up to 11-fold and the suppression of gamma interferon and IL-10, which are immunomodulatory cytokines with anti-viral and antitumor functions. Sympathetic nerve fibers within lymphoid organs such as spleen and lymph nodes, where most pathogen and immune system battles are waged, release neurohormones such as norepinephrine that can directly facilitate pathogen growth as well as alter white blood cell function (62). Hormonal response systems such as the HPAC, the sympathetic adrenal-medullary axis, and the hypothalamic-pituitary-ovarian axis when activated by PNI stressors release hormones such as cortisol, epinephrine, substance P, estradiol, testosterone, and so forth. High levels of these hormones serve to facilitate pathogen replication or reactivation in the case of latent infections and adversely affect T cell and cytokine balance. Recent epigenetic evidence suggests that stress hormones upregulate the immune response into generating increased levels of cytokines which enhance the expression of inflammation-controlling genes. Chronic stressors and the resulting increase in CRP, prostaglandins, thromboxanes, leukotrienes, and nitric oxide are an apparent attempt by the immune system to compensate for depression in one area of responsiveness by responding in a more exaggerated and aggressive fashion to challenges resulting in clinical manifestations of pain, chronic inflammation, and disease.
from the production of predominately Th1 cytokines to the production of predominately Th2 cytokines (58,59). Catecholamines and glucocorticoids fuel the switch from Th1 to Th2 cytokine production pattern resulting in decrements in lymphocyte numbers, cytotoxic T cell function, NK cell count, and viral surveillance. HIV viremia results from this sequence followed by an increased risk of HIV morbidity and mortality. The importance of a Th1 to Th2 cytokine shift demonstrated in HIV infections stems from the ability of Th1 cytokines to decrease the vulnerability of lymphocytes to apoptosis and inhibiting HIV replication, while Th2 cytokines increase the susceptibility to apoptosis and stimulate HIV replication (58,59). Research over the past two decades has elucidated a reciprocal relationship between the mind and body especially within a framework of anti-microbial immunity. Psychosocial factors including stress, depression, bereavement, medications, and so on create a pattern of interference on neuroendocrine function and outcomes of immunity. The switch from Th1 to Th2 is followed by increasing antibody titers to latent viral infections resulting in poorer viral immunosurveillance. Distressed individuals exhibit heightened sympathetic and HPAC activity further compromising antimicrobial immunity (60,61). Norepinephrine is associated with an increase in HIV replication up to 11-fold and the suppression of gamma interferon and IL-10, which are immunomodulatory cytokines with anti-viral and antitumor functions. Sympathetic nerve fibers within lymphoid organs such as spleen and lymph nodes, where most pathogen and immune system battles are waged, release neurohormones such as norepinephrine that can directly facilitate pathogen growth as well as alter white blood cell function (62). Hormonal response systems such as the HPAC, the sympathetic adrenal-medullary axis, and the hypothalamic-pituitary-ovarian axis when activated by PNI stressors release hormones such as cortisol, epinephrine, substance P, estradiol, testosterone, and so forth. High levels of these hormones serve to facilitate pathogen replication or reactivation in the case of latent infections and adversely affect T cell and cytokine balance. Recent epigenetic evidence suggests that stress hormones upregulate the immune response into generating increased levels of cytokines which enhance the expression of inflammation-controlling genes. Chronic stressors and the resulting increase in CRP, prostaglandins, thromboxanes, leukotrienes, and nitric oxide are an apparent attempt by the immune system to compensate for depression in one area of responsiveness by responding in a more exaggerated and aggressive fashion to challenges resulting in clinical manifestations of pain, chronic inflammation, and disease.
From a more balanced PNI perspective, the goal of the immune response is to eliminate an invading pathogen in the most effective and self-protecting means possible and restore harmony with oneself and one’s environment as quickly as possible. The process of externalizing a microbial infection often results in a constellation of specific and non-specific symptoms that define the clinical illness. The symptom profile is contingent upon the pathogen, the tissue colonized or infected, and the PNI status of the host. Respiratory viruses tend to elicit sore throat, runny nose, congestion, sneezing, and coughing. Enteric viruses tend to induce vomiting and diarrhea. Infections of the CNS can produce vomiting, headache, stiffness, and seizures. The coordinated efforts of Th1 cells and their cytokine array in response to viral infections, for example, serve to externalize and rid the body of the invader. Apart from the disease-specific symptoms, infections generally induce non-specific symptoms including fever, malaise, sleepiness, anorexia or increased appetite, anhedonia, and withdrawal from activities (55,62,63). Non-specific symptoms are referred to as sickness behavior and are induced by cytokines acting on the CNS (55,64). Researchers assert that sickness behavior represents an evolved strategy designed to maximize the chances of survival. Survival is dependent on the host’s ability to mount a vigorous defense and avoid contact with pathogens and predators that might exploit vulnerability. Initiation of a febrile response retards the reproductive capacity of pathogens and mobilizes the immune system for the externalization process. Sleeping, withdrawal from activities, appetite alteration, and so on are the means of conserving and refocusing energy. Cytokine induced non-specific symptoms as well as specific symptoms of mucus production, runny nose, nasal congestion, and so forth are seen as an adaptive response to infection rather than a pathological consequence of microbial invasion (62,64). Exposure to and the process of pathogen externalization can be viewed as a means by which the host can, for example, strengthen Th1 and corresponding neurologic and neuroendocrine functions especially after a prolonged period of stress and depressed PNI function. With a view toward optimizing and maintaining PNI balance and function, the traditional view of infectious disease, eliciting only pathology, needs to be replaced with a view toward infectious disease as a necessary means of adaptation to strengthen and rebalance neuroimmune function especially in the pediatric patient. This shift in consciousness will require better understanding and assessment of the host PNI to determine the defense capabilities and potential needs in the face of infectious disease as well as the appreciation of the variances in microbial virulence capacities.
THE ASSESSMENT OF THE IMMUNE SYSTEM
Consequential to the advances in PNI and corresponding clinical observations, is the need to develop an effective protocol for immune system assessment. The ability of
the clinician to monitor immune system and neuroendocrine function, especially in the pediatric patient, will be an invaluable tool for assessing the impact on the immune system of interference patterns as well as effectively evaluating intervention efficacy with the goal of obtaining and maintaining optimum immunity and neuroendocrine balance.
the clinician to monitor immune system and neuroendocrine function, especially in the pediatric patient, will be an invaluable tool for assessing the impact on the immune system of interference patterns as well as effectively evaluating intervention efficacy with the goal of obtaining and maintaining optimum immunity and neuroendocrine balance.
The clinician can assess the pediatric immune system qualitatively and quantitatively. A child presenting, for example, with a pattern of chronic viral respiratory and or gastrointestinal infections points to a deficient quality of Th1 CMI, the arm of the immune system necessary to appropriately manage viral infections. Children presenting with chronic allergy, asthma, and eczema symptoms point to a qualitative Th2 skewing of the immune system. Inference to Th2 skewing arises from a case history documenting the exposures to heavy metal, antibiotic, alcohol, vaccination, and so on, during the prenatal and neonatal periods. Quantitative immune assays provide validation of the qualitative evaluation and conclusion.
Standard laboratory analysis of lymphocyte counts and gamma globulin evaluation determine general immune competency. T helper cell analysis, T cell cytokine, and neuroendocrine profiles provide data necessary in evaluating the functional status of the immune and neuroendocrine systems. Vedhara and Wang (57) recommend quantitating T helper cell levels, T cell ratios, as well as T cell functional assessments to delineate immune imbalance and dysfunction. Assessment of multiple cytokines can provide an indirect evaluation of T helper cell function and T cell skewing (65). They advocate the widespread assessment of multiple cytokines in PNI and forsee the use of “arrays” that measure the changes in multitudes of genes and proteins. Laboratories currently provide comprehensive stool analyses to determine an individual’s sensitivity to dietary proteins, the presence of dysbiosis, and so on, and these test results serve as a starting point in generating a remedial plan to resolve immune imbalance, immune dysfunction, and excessive inflammation-type issues. Heavy-metal analyses are a valuable laboratory tool in determining prenatal and neonatal exposures to Hg, Al, and Pb that interfere with normal immune and neurologic functions. Technologies to evaluate T helper cell levels and cytokine profiles have been available in basic science since the mid-1990s and have been employed in determining the outcomes of vaccine strategies aimed at producing vaccines that induce the same T cell and cytokine responses that result from natural infection (19,66,67). The limiting factor of these technologies is expense, but systems such as the Affymetrix DNA array are being used to measure changes in the expression of over 10,000 genes in a single assay (57).
Costs of these valuable neuroimmune assays will become more reasonable when they become an integral part of standard pediatric wellness workups. The demand for all children to be vaccinated should also include a demand for all children to have baseline and periodic neuroimmune assessments to screen for and remediate imbalances before such imbalances progress to dysfunction.
The genetic and epigenetic foundations for optimum immune function, immunostasis, are laid down in-utero (1,2,68,69,70). Fetal or early programming refers to the concept that early environmental epigenetic factors can permanently organize or imprint physiological and behavioral systems. Recent studies are focusing on the patterns of PNI interference that may affect embryogenesis within the framework of pre-conception care (71).
PRENATAL AND NEONATAL INFLUENCES ON NEUROIMMUNE DEVELOPMENT
Research has established the adverse effects of prenatal alcohol exposure on neuroendocrine and immune functions generating broad-based impairments in both innate and adaptive immunity (69,72). Increased HPAC activity, resulting from fetal alcohol exposure, programs the immune system to be Th1-cell-mediated deficient with less adverse effect on Th2 immunity and propensity to adverse psychosocial stress for generations. Similar results have been found with fetal nutritional deficiencies (71,73,74), recreational drug exposure (75,76,77,78), and exposure to antibiotics (79,80,81). Studies focused on the effects of fetal ultrasound exposure have revealed the increased risk of neural, DNA, and blood damage resulting from ultrasound exposure in-utero (82,83,84,85). The effects of fetal ultrasound exposure on immunostasis have yet to be established. The role of increased sympathetic nervous tone on the innate and adaptive arms of the immune system has been well documented. As reviewed in Figure 10-1 the sympathetic nervous system drives the immune machinery into a depressed mode with consequences on the CNS and HPAC systems. The epigenetic effects of prolonged sympathetic tone are antigen processing cell dysfunction, and cytokine imbalance, and thereby it selectively suppresses the Th1 function and enhances the Th2 function (70,86,87). The resulting Th1-Th2 imbalance persists throughout the life of the individual and increases the incidence of infectious, malignant, and chronic degenerative disease (43,88,89). The role of spinal lesions or subluxations in increasing the sympathetic tone has been documented (24,25,26,27,28,90,91,92,93). This was originally described as the neurodystropic effect (94), and provides another source of epigenetic interference impacting postnatal immune
competence. The role of spinal lesions in influencing fetal PNI development and function needs elucidation.
competence. The role of spinal lesions in influencing fetal PNI development and function needs elucidation.
Maternal-fetal immune competence is normally biased toward humoral immunity and cellular immunity naturally suppressed to prevent rejection responses (95). Th2 responses with the production of cytokines IL-3, IL-4, and IL-5 dominate over Th1 responses and cytokines such as IL-2 and gamma interferon. Evidence suggests the suppressive role of progesterone and regulatory T cells in the normal Th2 skewing seen during pregnancy (96,97,98). The role of additional stressors such as increased sympathetic tone, nutritional deficiencies, and chemical and drug exposures on reinforcing and permanently skewing immune balance needs further study.
THE BIRTH PROCESS, NEONATAL FEEDING, AND ENVIRONMENTAL EFFECTS ON IMMUNOSTASIS
Studies have revealed that the birth process, colostrum intake, breast feeding, and exposure to the environment are factors directing the immature, neonatal immune system toward the more mature Th1 orientation. Evidence reveals the health differences between
natural versus cesarean birth (99,100), and breast-fed versus formula-fed babies (101,102,103,104), reflecting the differences in Th1-Th2 immunostasis (105,106). Travel through the birth canal exposes the neonate to the normal bacterial flora that colonize the neonate’s gastrointestinal tract (107,108). Colostrum and breast milk are rich in cytokines essential for Th1 stimulation (109,110). Passive immunity consisting of maternal Igs passing through the placenta and in the breast milk assists the embryo and neonate in humoral immune defense. The presence of an appropriate, probiotic environment stimulates Th1 immune potential. Maternal prebiotic nutrients (111) and probiotics in the breast milk (112,113) continue this Th1 stimulatory process during the post-natal period. Maternal prebiotic nutrients in the breast milk consist of carbohydrates that preferentially favor the growth of the appropriate bowel flora. Neuroendocrine, immune, digestive, and growth factors found in the breast milk appear to be regulated by post-natal feedback mechanisms. Cytokines present in the nursing child’s saliva appear to be a modulator of breast milk contents (114,115,116,117,118). Studies need to elucidate the role of the maternal sense of smell, as well as other sensory feedback mechanisms, directed toward the nursling serving to regulate breast milk contents. Inappropriate bowel bacteria (dysbiosis) and formula-based carbohydrates favor toxic fermentation-type processes in the pediatric bowel resulting in bowel inflammation, colic, and a skewing toward Th2-type immune responsiveness (119,120,121,122,123). The use of epidural analgesia during the birth process correlates with negative breast-feeding outcomes (124,125). The infant’s exposure to the environment of gram-negative bacteria, viruses, and fungi serves to further direct its maturing immune system to immunostatic Th1 levels (126,127). Several lines of evidence point to childhood infections providing significant protection from Th2-mediated asthma and atopy (106,110). The lack of appropriate microbial stimulation of sufficient intensity in early life affects the maturation of the immune system by contributing to a Th2-biased balance to immune responsiveness. The pattern of cesarean section, lack of colostrum and breast milk, vaccination, and lack of microbial stimulation serves to condition the maturing infant immune system to becoming Th2 dominant (2,101). Th2 dominant immune responsiveness suppresses Th1 function rendering the child susceptible to viral infections (128,129). When viral infections do occur, many infants are managed with courses of broad-spectrum antibiotics. These antibiotics are directed against bacterial infections and are ineffective against viruses. Normal bowel flora are eliminated by indiscriminate antibiotic use as well as Th1 cell activity, proportionately. The ability of these interferences as well as the impact of genetic and epigenetic factors on lifelong immune response programming requires further investigation.
natural versus cesarean birth (99,100), and breast-fed versus formula-fed babies (101,102,103,104), reflecting the differences in Th1-Th2 immunostasis (105,106). Travel through the birth canal exposes the neonate to the normal bacterial flora that colonize the neonate’s gastrointestinal tract (107,108). Colostrum and breast milk are rich in cytokines essential for Th1 stimulation (109,110). Passive immunity consisting of maternal Igs passing through the placenta and in the breast milk assists the embryo and neonate in humoral immune defense. The presence of an appropriate, probiotic environment stimulates Th1 immune potential. Maternal prebiotic nutrients (111) and probiotics in the breast milk (112,113) continue this Th1 stimulatory process during the post-natal period. Maternal prebiotic nutrients in the breast milk consist of carbohydrates that preferentially favor the growth of the appropriate bowel flora. Neuroendocrine, immune, digestive, and growth factors found in the breast milk appear to be regulated by post-natal feedback mechanisms. Cytokines present in the nursing child’s saliva appear to be a modulator of breast milk contents (114,115,116,117,118). Studies need to elucidate the role of the maternal sense of smell, as well as other sensory feedback mechanisms, directed toward the nursling serving to regulate breast milk contents. Inappropriate bowel bacteria (dysbiosis) and formula-based carbohydrates favor toxic fermentation-type processes in the pediatric bowel resulting in bowel inflammation, colic, and a skewing toward Th2-type immune responsiveness (119,120,121,122,123). The use of epidural analgesia during the birth process correlates with negative breast-feeding outcomes (124,125). The infant’s exposure to the environment of gram-negative bacteria, viruses, and fungi serves to further direct its maturing immune system to immunostatic Th1 levels (126,127). Several lines of evidence point to childhood infections providing significant protection from Th2-mediated asthma and atopy (106,110). The lack of appropriate microbial stimulation of sufficient intensity in early life affects the maturation of the immune system by contributing to a Th2-biased balance to immune responsiveness. The pattern of cesarean section, lack of colostrum and breast milk, vaccination, and lack of microbial stimulation serves to condition the maturing infant immune system to becoming Th2 dominant (2,101). Th2 dominant immune responsiveness suppresses Th1 function rendering the child susceptible to viral infections (128,129). When viral infections do occur, many infants are managed with courses of broad-spectrum antibiotics. These antibiotics are directed against bacterial infections and are ineffective against viruses. Normal bowel flora are eliminated by indiscriminate antibiotic use as well as Th1 cell activity, proportionately. The ability of these interferences as well as the impact of genetic and epigenetic factors on lifelong immune response programming requires further investigation.
THE ROLE OF VACCINATIONS ON NEUROIMMUNE FUNCTION
Another variable involved in skewing immune competence toward Th2 with the suppression of Th1 is the current spectrum of neonatal and pediatric vaccinations (130,131,132). Observations stemming from the use of a tuberculosis vaccine in the early 1990s led to the hypothesis of different T cell subsets being dominant in natural tuberculosis infections as compared to an injected tuberculosis vaccine (133). Natural infection with tuberculosis results in a Th1- and macrophagedominant response to externalize and recover from the infection. TB vaccinations skew responses to a humoral, Th2-based response-eliciting antibodies that are not effective in the resolution of infection. Assessment of childhood vaccines, especially those vaccines directed against viral diseases, also resulted in the vaccines-generating responses skewed to humoral Th2-based responses in contrast to the Th1 responses found among those experiencing the natural infections (19,66). The landmark 1995 Golding and Scott (66) publication brought focus to vaccine strategies targeting T cell populations. The authors asserted the understanding that Th1 CMI afforded protection against intracellular infections such as viruses in contrast to Th2 humoral responses that increase susceptibility to intracellular infections. They proposed using different adjuvants, conjugation methods, routes of administration, cytokine incorporation, and different forms of vaccine components as strategies to arrive at the required T cell bias. Adjuvants are potent, non-specific substances added to vaccines to increase the immunogenicity of the vaccine antigen (9,134,135,136). Aluminum potassium sulfate (alum) is the current vaccine adjuvant used to generate high-titer Th2 antibody responses. The alum salt generates a precipitation with the vaccine antigen at the injection site resulting in a slower release of antigen from the injection site (9,67,137). Exposure to the antigen increases from a few days without the adjuvant to several weeks with the adjuvant. Squalenes are oils normally found within the nervous system and are used as adjuvants in vaccines. Squalene use in vaccines designed for the military is associated with the Gulf War syndrome and induces autoimmunity in animal systems (138,139). The resulting Th2 response induces a temporary immunity to the desired microbe or virulence factor that requires periodic boosting every 3 to 5 years to maintain protective antibody levels. The length of time protective antibody levels are maintained
varies among individuals. The period of temporary immunity in which protective antibody levels are present is followed by a period of deferred susceptibility to the specific microbe or virulence factor. Injected vaccines preclude the development of mucosal immunity in the respiratory, gastrointestinal, and genitourinary tracts. Mucosal immunity acquired from natural infectious processes is a vital component of natural acquired immunity. Vaccines utilizing routes of administration analogous to naturally acquired infections engage the natural dendritic cell process and have shown promise in generating the appropriate Th cell bias (131). Natural immunity to the viral diseases of childhood generates a Th1 cell bias providing lifelong immunity. The use of Freund’s complete adjuvant and the cytokine adjuvant, IL-12 (140,141), stimulate the required Th1 response, but expense and host toxicity preclude the use of these agents (9). Vaccines incorporating the routes of administration that mimic the natural infectious process induce dendritic cell activity necessary for appropriate immune responses (131). However, the risks of releasing live vaccine organisms into the environment and the lack of accurate quantitation of vaccine dosage acquired by the recipient preclude this strategy. Conjugation entails coupling a low-molecular-weight vaccine antigen to a large-molecular-weight carrier to increase the immunogenicity of the vaccine entity (142,143). Conjugation methodology has been used with the hepatitis, hemophilus, and pneumococcus vaccines, and attempts to alter protein carriers strategically fail to elicit the required Th1 bias. To date the most attempted strategies have failed to elicit the required T cell bias to the vaccine component leaving the most advantageous T cell response that is protective. For example, host immune responses elicited by the current viral vaccine strategies substitute the natural required Th1-biased response with the protective, artificial Th2 response (144



varies among individuals. The period of temporary immunity in which protective antibody levels are present is followed by a period of deferred susceptibility to the specific microbe or virulence factor. Injected vaccines preclude the development of mucosal immunity in the respiratory, gastrointestinal, and genitourinary tracts. Mucosal immunity acquired from natural infectious processes is a vital component of natural acquired immunity. Vaccines utilizing routes of administration analogous to naturally acquired infections engage the natural dendritic cell process and have shown promise in generating the appropriate Th cell bias (131). Natural immunity to the viral diseases of childhood generates a Th1 cell bias providing lifelong immunity. The use of Freund’s complete adjuvant and the cytokine adjuvant, IL-12 (140,141), stimulate the required Th1 response, but expense and host toxicity preclude the use of these agents (9). Vaccines incorporating the routes of administration that mimic the natural infectious process induce dendritic cell activity necessary for appropriate immune responses (131). However, the risks of releasing live vaccine organisms into the environment and the lack of accurate quantitation of vaccine dosage acquired by the recipient preclude this strategy. Conjugation entails coupling a low-molecular-weight vaccine antigen to a large-molecular-weight carrier to increase the immunogenicity of the vaccine entity (142,143). Conjugation methodology has been used with the hepatitis, hemophilus, and pneumococcus vaccines, and attempts to alter protein carriers strategically fail to elicit the required Th1 bias. To date the most attempted strategies have failed to elicit the required T cell bias to the vaccine component leaving the most advantageous T cell response that is protective. For example, host immune responses elicited by the current viral vaccine strategies substitute the natural required Th1-biased response with the protective, artificial Th2 response (144
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree