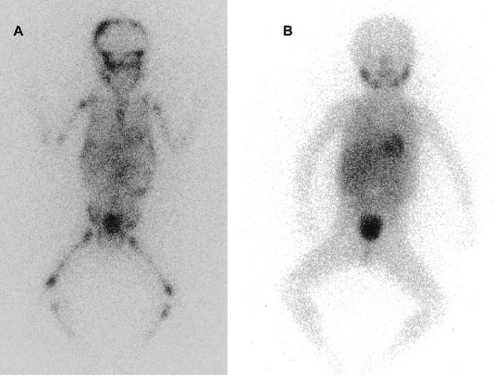Neuroblastoma
Andrew M. Davidoff
Department of Surgery, St. Jude Children’s Research Hospital, University of Tennessee Health Science Center, Memphis, Tennessee 38105.
Neuroblastoma is the most common solid extracranial malignancy of childhood and the most common malignant tumor in infants (i.e., patients younger than 1 year) (1); the total incidence is 1 per 100,000 children in the United States. Neuroblastoma represents 7% to 10% of all malignancies diagnosed in pediatric patients younger than 15 years of age and is responsible for approximately 15% of all pediatric cancer deaths (2). However, neuroblastoma is a heterogeneous disease (3); tumors can spontaneously regress or mature, or display a very aggressive, malignant phenotype. Because of these unique characteristics, neuroblastoma has been of great interest to both clinicians and basic scientists. Progress in molecular and cellular biology and immunology in the past 10 years has contributed greatly to a better understanding of this disease; however, this progress has not significantly altered the clinical outcome for patients with neuroblastoma. Although the prognosis for patients with advanced-stage neuroblastoma has improved somewhat since the mid-1980s, the long-term survival of these patients is still dismal.
The etiology of neuroblastoma is currently unknown, and no environmental factors have been linked to its development. The disease generally occurs sporadically, but familial neuroblastoma does occur, in about 2% of the cases. Interestingly, substantial biologic and clinical heterogeneity is often observed in familial cases (4). Neuroblastoma has also been reported in infants with Beckwith-Wiedemann syndrome, Hirschsprung’s disease, or fetal alcohol syndrome and in the offspring of mothers taking phenylhydantoin for seizure disorders (5,6,7,8).
The treatment of neuroblastoma requires a multidisciplinary approach. Although surgical resection may be the only therapy required for patients at “low risk” of disease recurrence, the surgeon provides but one element of the modern multimodal treatment of children with “high-risk” disease. Pediatric oncologists, radiation therapists, and bone marrow transplantation (BMT) specialists are among the other important members of the pediatric oncology team. The therapy for patients with neuroblastoma is generally driven by clinical research protocols. Many protocols are sponsored by the Children’s Oncology Group (COG) or by the larger children’s hospitals, working individually or in small groups, and are often based on the results of previous trials conducted by the two legacy pediatric cancer cooperative groups, the Pediatric Oncology Group (POG) and the Children’s Cancer Group (CCG).
PATHOLOGY
Neuroblastoma is an embryonal tumor of the sympathetic nervous system. These tumors arise during fetal or early postnatal life from sympathetic cells (sympathogonia) derived from the neural crest. Therefore, tumors can originate anywhere along the path that neural crest cells migrate, including the adrenal medulla, paraspinal sympathetic ganglia, and sympathetic paraganglia, such as the organ of Zuckerkandl. The German pathologist Rudolph Virchow is generally credited with being the first to describe the histologic appearance of what is now known as neuroblastoma in his 1864 article entitled, “Hyperplasia of the pineal and suprarenal glands” (9). The first to use the term neuroblastoma was James Homer Wright who in 1910 described the classic appearance of rosettes of tumor cells around central neural fibrils. He also noted the association between the common sites of tumor development and the pattern of migration of primitive neural cells (10).
The gross appearance of neuroblastoma depends on its location, degree of maturation, and whether it has undergone necrosis or hemorrhage. Classically, neuroblastomas are purple, highly vascular, and friable. The tumor often becomes more nodular and frequently appears fleshy white with maturation or in response to therapy. Larger tumors frequently have calcifications resulting from necrosis or hemorrhage.
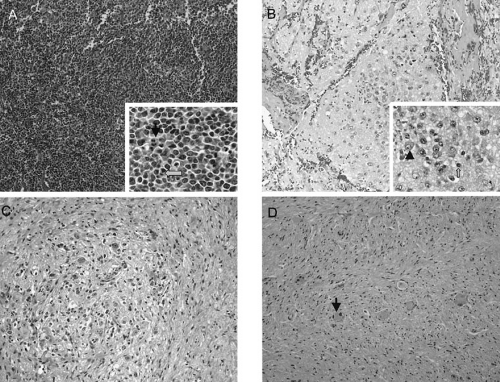
As one of the “small, round blue cell” tumors of infancy and childhood, neuroblastoma, particularly when undifferentiated, must be distinguished from other neoplasms of this group [e.g., Ewing’s sarcoma family of tumors (ESFT), non-Hodgkin’s lymphoma, and rhabdomyosarcoma]. Neuroblastoma is distinguished histologically by the presence of neuritic processes (neuropil) and Homer Wright rosettes (neuroblasts surrounding eosinophilic neuropil). Scattered ganglion cells or immature chromaffin cells may also be seen. Immunohistochemical analysis usually generates positive staining when antibodies to neuroblastoma-specific antigens such as synaptophysin, neuron-specific enolase, and chromogranin are used, and is negative when antibodies to actin, desmin, cytokeritin, leukocyte common antigen, vimentin, and CD99 are used. At the microscopic level, the appearance of the tumor cells may vary from undifferentiated cells to fully mature ganglion cells. In addition, neuroblastomas have variable degrees of Schwannian cell stroma, which is the reactive nonneoplastic tissue recruited by the tumor cells. This stroma is intermixed, to a greater or lesser degree, as wavy bundles and sheets of spindle cells throughout a tumor and produces antiproliferative and differentiation-inducing factors that are crucial to neuronal differentiation (11,12). In addition, the Schwannian stroma appears to produce a variety of antiangiogenic factors, including pigment epithelium-derived factor (13) and secreted protein acidic and rich in cysteine (14). Histopathologic variables among neuroblastic tumors include the degree of differentiation, maturation, lymphoid infiltration, calcification, anaplasia, necrosis, mitotic activity, neurofibrillary material (neuropil), and the presence of multinucleate cells.
Neuroblastoma is characterized by several unique clinical behaviors, including the secretion of catecholamine products and the potential to regress or mature; this may occur spontaneously, or with stimulation. Small nodules of primitive neuroblasts are routinely found in the developing adrenal gland, even during the early postnatal period. Beckwith and Perrin described microscopic nodules that they termed neuroblastoma in situ in the adrenals of infants undergoing autopsy following death from nonmalignancy-related causes (15). The incidence of this finding was more than 200-fold greater than the clinical incidence of neuroblastoma, suggesting that perhaps many neuroblastomas spontaneously regress or mature into lesions that never become clinically apparent.
The process of involution is well described during embryonic life, especially in the developing central and peripheral nervous systems. Although initially believed to be mediated by the immune system, the process of involution may be the result of the withdrawal of neurotrophic maintenance factors such as nerve growth factor. Clinically apparent neuroblastoma can also regress or spontaneously mature, but the mechanism(s) of neuroblastoma maturation and regression remain unknown.
Shimada Classification
Shimada and colleagues (16) developed an age-linked classification system of neuroblastic tumors that is based on the morphology of the primary tumor. Neuroblastomas were divided into two prognostic subgroups, favorable histology and unfavorable histology, based on histologic characteristics, including the degree of neuroblast differentiation, the nuclear morphology of neuroblastic cells (mitosis-karyorrhexis index [MKI]), and the presence or absence of surrounding Schwannian stroma (17). This classification system has become widely accepted and has proven to be useful as an independent predictor of disease outcome.
A simpler system was devised in 1984, known as the International Neuroblastoma Pathology Classification (INPC), a classification scheme that included modifications of the Shimada system (18). The INPC is based mainly on morphologic changes associated with the maturational sequence of neuroblastic tumors (19). It is an age-linked classification that depends on the differentiation grade of the neuroblasts, the cellular turnover index (MKI), and the presence or absence of Schwannian stroma.
The INPC classifies neuroblastic tumors into three morphologic categories: neuroblastoma, ganglioneuroblastoma, and ganglioneuroma (Fig. 36-1). Neuroblastomas are, by definition, Schwannian stroma poor (less than 50% of the tumor tissue) and can be subtyped as undifferentiated (requires supplemental diagnostic methods such as immunohistochemistry, electron microscopy, or cytogenetics, and neuropil is not present); poorly differentiated (less than 5% of tumor cells have features of differentiation, and neuropil is present); or differentiating (more than 5% of tumor cells show differentiation toward ganglion cells). To classify a cell as a differentiating neuroblast, there must be synchronous differentiation of the nucleus (an enlarged, eccentric nucleus with a vesicular chromatin pattern and a single prominent nucleolus) and eosinophilic cytoplasm (19).
Stroma-rich neuroblastic tumors are classified as either ganglioneuroblastomas or ganglioneuromas. Ganglioneuroblastomas contain cells that are transitioning toward differentiation but are not completely differentiated/mature and have residual microscopic foci of neuroblastic cells (less than 50% of the total volume) intermixed or randomly distributed throughout the tumor; ganglioneuromas contain either maturing cells (i.e., those that contain a minor component of scattered differentiating neuroblasts not forming distinctive nests), or mature cells, which lack any neuroblastomatous component. Ganglioneuroblastomas are further divided into “intermixed” and “nodular” subtypes. The distinction is important because of the significantly poorer prognosis associated with the latter subtype, in which the neuroblastic clones that
comprise the grossly distinct nodules are responsible for the aggressive phenotype for this subtype (20). The INPC staging system is summarized in Table 36-1.
comprise the grossly distinct nodules are responsible for the aggressive phenotype for this subtype (20). The INPC staging system is summarized in Table 36-1.
The importance of this histopathologic classification was confirmed in a large, retrospective analysis reported by Shimada et al. (21). The INPC classification of tumor histology provided independent prognostic information that was able to distinguish patients with tumors of favorable histology (probability of 5-year event-free survival [EFS], 90.8%) from those with tumors of unfavorable histology (EFS, 31.2%). Histopathology has been included in the current multifactorial risk stratification for patients with neuroblastoma; this determination is particularly important in patients who are older than 1 year and have localized disease. Because the histopathologic pattern within a single tumor can be heterogeneous, Shimada has recommended analyzing representative sections from at least 1 cm3 of viable, nonnecrotic tissue to determine histopathologic classification. This recommendation has significant implications for the surgeon considering whether to perform an “open” or percutaneous core needle biopsy on a tumor suspected of being a localized neuroblastoma in a child older than 1 year of age. Finally, the prognostic value of assessing the histopathology of a neuroblastoma after chemotherapy or radiation therapy has not been validated.
CLINICAL PRESENTATION
Patients with neuroblastoma usually present with signs and symptoms that reflect the primary site and extent of disease, although localized disease is often asymptomatic.
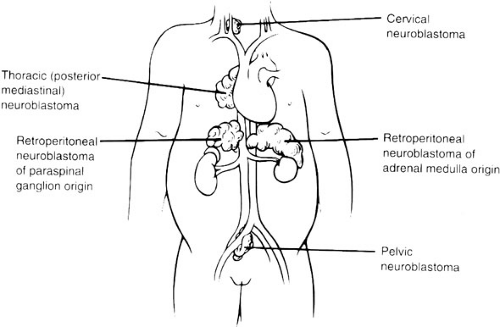
Because 75% of neuroblastoma occurs in the abdominal cavity (50% occur in the adrenal gland, 25% occur elsewhere in the retroperitoneum), an abdominal mass detected on physical examination is a common clinical feature, as is the complaint of abdominal pain. Other primary sites of neuroblastoma include the posterior mediastinum (20%), the cervical region (1%), and the pelvis (4%) (Organ of Zuckerkandel) (Fig. 36-2). Respiratory
distress or dysphagia may be a reflection of a thoracic tumor. Altered defecation or urination may be caused by mechanical compression of a pelvic tumor or by spinal cord compression by a paraspinal tumor. Spinal cord compression may also present with an altered gait. A tumor in the neck or upper thorax can produce Horner’s syndrome (ptosis, miosis, and anhydrosis), enophthalmos, and heterochromia of the iris. Acute cerebellar ataxia has also been observed, characterized by the dancing-eye syndrome, which includes opsoclonus-myoclonus, and chaotic nystagmus. Two-thirds of these cases occur in infants with mediastinal primary tumors (22,23). Additional signs and symptoms that reflect excessive catecholamine or vasoactive intestinal polypeptide secretion include diarrhea, weight loss, and hypertension.
Because 75% of neuroblastoma occurs in the abdominal cavity (50% occur in the adrenal gland, 25% occur elsewhere in the retroperitoneum), an abdominal mass detected on physical examination is a common clinical feature, as is the complaint of abdominal pain. Other primary sites of neuroblastoma include the posterior mediastinum (20%), the cervical region (1%), and the pelvis (4%) (Organ of Zuckerkandel) (Fig. 36-2). Respiratory
distress or dysphagia may be a reflection of a thoracic tumor. Altered defecation or urination may be caused by mechanical compression of a pelvic tumor or by spinal cord compression by a paraspinal tumor. Spinal cord compression may also present with an altered gait. A tumor in the neck or upper thorax can produce Horner’s syndrome (ptosis, miosis, and anhydrosis), enophthalmos, and heterochromia of the iris. Acute cerebellar ataxia has also been observed, characterized by the dancing-eye syndrome, which includes opsoclonus-myoclonus, and chaotic nystagmus. Two-thirds of these cases occur in infants with mediastinal primary tumors (22,23). Additional signs and symptoms that reflect excessive catecholamine or vasoactive intestinal polypeptide secretion include diarrhea, weight loss, and hypertension.
TABLE 36-1 Prognostic Evaluation of Neuroblastic Tumors According to the International Neuroblastoma Pathology Classification (Shimada System). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
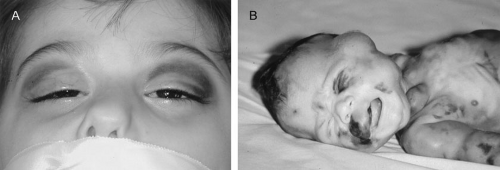
The population-based distribution of neuroblastoma stage at presentation is shown in Table 36-2. More than 40% of patients have metastatic disease at diagnosis. These patients are often quite ill and have systemic symptoms caused by widespread disease. Neuroblastoma in older patients has a pattern of metastatic disease in which metastasis of the bone marrow, lymph nodes, and bone predominate. The frequency of involvement of distant sites is shown in Table 36-3. These metastases may manifest as bone pain (bone metastases) or anemia (bone marrow infiltration). The brain, spinal cord, heart, and lungs are rare sites of metastases, except with advanced, end-stage disease. Metastatic disease may be also associated with “black eyes” (also referred to as “raccoon eyes”) as a result of retroorbital venous plexus spread (Fig. 36-3A). This is an ominous physical sign, as is the presence of a limp in children without a history of head or extremity trauma. Infants with metastatic neuroblastoma may have stage 4S disease, which, by definition, is a localized primary tumor in patients younger than 1 year of age, with dissemination limited to skin, liver, or bone marrow (less than 10% of nucleated cells). These patients may present with “blueberry muffin” cutaneous lesions (Fig. 36-3B), respiratory
distress secondary to massive hepatomegaly, and anemia secondary to bone marrow disease.
distress secondary to massive hepatomegaly, and anemia secondary to bone marrow disease.
TABLE 36-2 Percentage Distribution of Neuroblastoma Cases by Age and Stage. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 36-3 Sites of Metastasis at Diagnosis for Patients with Stage IVS, Stage IV (<1 year of age), and Stage IV (≥1 year of age). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The diagnosis of neuroblastoma is generally made by histopathologic evaluation of primary or metastatic tumor tissue, or by the demonstration of tumor cells in the bone marrow and elevated levels of urinary catecholamines. Recommendations for the assessment of the extent of disease are outlined in Table 36-4.
Laboratory Findings
Lactate Dehydrogenase
Despite its lack of specificity, serum lactate dehydrogenase (LDH) can have great prognostic significance. High serum levels of LDH reflect high proliferative activity or large tumor burden (24), and an LDH level higher than 1500 IU per L appears to be associated with a poor prognosis (25). Thus, LDH can be used to monitor disease activity or the response to therapy.
Ferritin
High levels of serum ferritin (more than 150 ng per mL) may also reflect a large tumor burden or rapid tumor progression. Elevated serum ferritin is often seen in advanced-stage neuroblastomas and indicates a poor prognosis (26), although levels often return to normal during clinical remission.
Neuron-specific Enolase
Neuron-specific enolase (NSE) is another useful prognostic marker of advanced-stage neuroblastomas. The incidence of elevated NSE levels increases with stage (27). A serum level of NSE greater than 100 ng per mL is associated with a poor outcome. NSE has been reported to correlate with tumor burden (28), suggesting its reliability as a marker of disease course.
Catecholamine Metabolites
As mentioned previously, neuroblastoma is characterized by the relatively unique capacity for the secretion of
catecholamine products, the metabolites of which can be detected in the urine of more than 90% of patients with neuroblastoma. Thus, a urine specimen is of clinical value in diagnosing neuroblastoma and determining the response to therapy.
catecholamine products, the metabolites of which can be detected in the urine of more than 90% of patients with neuroblastoma. Thus, a urine specimen is of clinical value in diagnosing neuroblastoma and determining the response to therapy.
TABLE 36-4 Assessment of Extent of Disease. | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
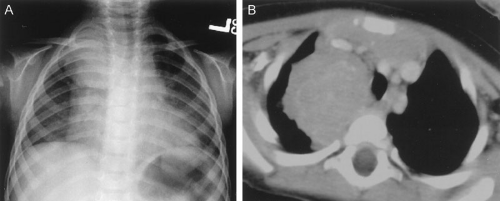 FIGURE 36-4. Posterior mediastinal/thoracic neuroblastoma as seen on (A) chest radiograph and (B) chest CT scan. |
Homovanillic acid (HVA) and vanillylmandelic acid (VMA) can be measured in the urine; high levels (i.e., three standard deviations or more above the mean per creatinine, corrected for age) (29) can be used to confirm the diagnosis of neuroblastoma. Documentation of this is required if the diagnosis is being made by the identification of neuroblasts in the bone marrow. Urinary levels of these two catabolites can also be used as markers of tumor progression and serve as a surrogate prognostic indicator.
Neuroblastomas with amplification of the MYCN gene tend to be dopaminergic; therefore, patients with this subtype of neuroblastoma have an elevated urinary HVA level, but normal norepinepherine and VMA levels. The measurement of urinary dopamine is recommended for detecting so-called nonsecretory neuroblastoma (30). Monitoring urinary catecholamine secretion during follow-up can detect a relapse of neuroblastoma, whereas mild elevations of the products may indicate remission or maturation of residual elements (31). Random urine samples are preferable to 24-hour urine estimations for younger children (32).
Radiographic Imaging
Standard Radiographs
Chest radiography can be a useful tool for demonstrating the presence of a posterior mediastinal mass, which in a child is usually a thoracic neuroblastoma. A POG study demonstrated that a mediastinal mass was discovered on incidental chest radiographs in almost one-half of patients with thoracic neuroblastoma who had symptoms seemingly unrelated to their tumors (Fig. 36-4) (33). Abdominal radiography is less often the modality by which a neuroblastoma is discovered; however, as many as one-half of abdominal neuroblastomas are detectable as a mass with fine calcification.
Ultrasonography
Although ultrasonography is the modality most often used during the initial assessment of a suspected abdominal mass, its sensitivity and accuracy are less than that of computed tomography (CT) or magnetic resonance imaging (MRI) for diagnosing neuoblastoma (34). These latter modalities are generally used after screening with ultrasound to assist in generating a differential diagnosis and for further anatomic definition once the presence of a mass has been confirmed. Ultrasonography can demonstrate tumor involvement with major vessels and organs, and it can provide real-time three-dimensional information regarding their relation to the tumor. Color Doppler ultrasound can supply further information about vascular encasement within a tumor (35). In addition, Doppler ultrasound offers a better understanding of the biologic behavior of the tumor by analyzing malignant neovasculature and how it is affected by therapy (36).
Computed Tomography
CT remains a useful, commonly used modality for the evaluation of neuroblastoma. It can demonstrate calcification in almost 85% of neuroblastomas, and intraspinal extension of the tumor can be determined on contrast-enhanced
CT (37). Overall, contrast-enhanced CT has been reported to be 82% accurate in defining neuroblastoma extent, with the accuracy increasing to nearly 97% when performed with a bone scan (38). Although some consider CT to have been supplanted by MRI, others still consider it to be the image modality of choice for patients with neuroblastoma, especially when used in conjunction with bone scintigraphy (39).
CT (37). Overall, contrast-enhanced CT has been reported to be 82% accurate in defining neuroblastoma extent, with the accuracy increasing to nearly 97% when performed with a bone scan (38). Although some consider CT to have been supplanted by MRI, others still consider it to be the image modality of choice for patients with neuroblastoma, especially when used in conjunction with bone scintigraphy (39).
Magnetic Resonance Imaging
MRI is becoming the most useful and most sensitive imaging modality for the diagnosis and staging of neuroblastoma (34,40). MRI appears to be more accurate than CT for detection of stage 4 disease: the sensitivity of MRI is 83% and that of CT is 43%, and the specificity of MRI is 97% and that of CT is 88% (40). Metastases of the bone and bone marrow, in particular, are better detected by MRI, as is intraspinal tumor extension (40). When considering skeletal metastases alone, MRI and bone scan have been shown to be equivalent (40). Encasement of major vessels can be better defined by MRI than CT, especially with the use of MR angiography. MRI in the coronal plane is suitable for routine assessment of the whole body from the neck to the pelvis. Evaluating the utility of whole-body MRI, perhaps performed in conjunction with a functional imaging study such as positron emission tomography, is being considered for future clinical staging studies. CT and MRI are not very accurate for staging localized disease; however, the sensitivity of T1- and T2-weighted MR images is 100% for detecting neuroblastomas in infants identified by mass screening.
Metaiodobenzylguanidine Imaging
Metaiodobenzylguanidine (MIBG) is transported to and stored in the distal storage granules of chromaffin cells in the same way as norepinephrine. MIBG has been used for scintigraphic imaging of neuroblastoma. The MIBG scintiscan is the imaging study of choice in evaluating the involvement of bone and bone marrow by neuroblastoma (Fig. 36-5), having largely replaced technetium-99m methylene diphosphonate. (99mTc-MDP) bone scans, which are generally inferior to MIBG in detecting neuroblastomas with skeletal or extraskeletal involvement. In addition, monitoring MDP-avid neuroblastomas by bone scintigraphy often results in false-positive imaging for months after tumor remission.
At this time, the availability of MIBG scintiscans is still being somewhat limited; 99mTc-MDP bone scanning is the second choice if MIBG imaging is not available or does not visualize known disease (29,41). Iodine-131 (131I) or iodine-123 (123I) can be used to label MIBG. 123I-MIBG supplies a reduced absorbed radiation dosage and superior spatial resolution (42). The reported sensitivity of MIBG in the detection of neuroblastomas with metastases of the bone and bone marrow is 82%, and the specificity is 91% (43). Primary tumors and lymph node metastases are also detectable. MIBG can demonstrate more sites of tumor involvement in bone and bone marrow than either bone scintigraphy or standard radiography (43). However, false-negative MIBG scans have been seen in cases in which the bone scintigraphy was positive (41).
MIBG uptake indicates better prognoses in infants who have metastatic neuroblastoma (44). MIBG also appears to be useful for monitoring the response to therapy. Matthay et al. showed a correlation between early metastatic response by 123I-MIBG scintigraphy with overall response and EFS in patients with stage 4 disease (45).
Finally, immunoscintigraphy may be even better than MIBG scintigraphy. Reuland et al. reported that immunoscintigraphy with the chimeric antiganglioside antibody, ch14.18, labeled with 99mTc was more sensitive than MIBG for the diagnosis of local tumor recurrence and for the earlier detection of new metastases (46). Although immunoscintigraphy is not currently widely available, it may be a useful surveillance modality in the future.
Bone Marrow Examination
Bone marrow biopsy has been regarded as a routine and important method for detecting bone marrow involvement with neuroblastoma. Both aspiration and trephine biopsy should be performed, although the latter has better diagnostic value. To collect more accurate information, taking specimens from multiple sites is recommended. Immunohistochemical staining with antibodies such as antiganglioside GD2, S-100, NSE, and ferritin is also useful for reducing the number of false-negative cases (47). Because biopsy is invasive and painful, noninvasive alternatives are being tested. Studies have suggested the superiority of MRI (48) and MIBG scintigraphy (49) over bone marrow biopsy in detecting bone marrow infiltration by neuroblastoma; however, the specificity of these modalities requires further evaluation.
Differential Diagnosis
Making a correct clinical diagnosis of neuroblastoma can be difficult because patients present with such diverse symptoms. For example, acute cerebellar ataxia with opsoclonus-myoclonus can be mistaken for a primary neurologic disease. Widespread bone involvement may resemble nonneoplastic bone disease, such as osteomyelitis or rheumatoid arthritis, or be associated with systemic inflammatory changes. Symptoms referable to vasoactive intestinal polypeptide secretion (i.e., diarrhea) can be misinterpreted as symptoms of an enteric infection or inflammatory bowel disease.
Histologically, undifferentiated, small round blue cell neuroblastomas may be hard to distinguish from
rhabdomyosarcoma, primitive neuroectodermal tumors, ESFT, non-Hodgkin’s lymphoma, or acute megakaryoblastic leukemia. The use of a panel of specific antibodies (48), mentioned previously, can facilitate histologic differentiation. Electron microscopy can also be used to detect both neurosecretory dense-core granules in the peripheral cytoplasm and neural processes containing microtubules. The criteria for diagnosis of neuroblastoma recommended in the International Neuroblastoma Staging System (INSS) emphasize histologic assessment, as well as measurement of urine or serum catecholamine products (Table 36-5).
rhabdomyosarcoma, primitive neuroectodermal tumors, ESFT, non-Hodgkin’s lymphoma, or acute megakaryoblastic leukemia. The use of a panel of specific antibodies (48), mentioned previously, can facilitate histologic differentiation. Electron microscopy can also be used to detect both neurosecretory dense-core granules in the peripheral cytoplasm and neural processes containing microtubules. The criteria for diagnosis of neuroblastoma recommended in the International Neuroblastoma Staging System (INSS) emphasize histologic assessment, as well as measurement of urine or serum catecholamine products (Table 36-5).
Tumor Staging
In the past, two primary systems have been used to stage neuroblastoma, in addition to the tumor-nodes-metastasis classification proposed by the International Union Against Cancer. These are the Evans classification used by the former CCG institutions (50) and the St. Jude Children’s Research Hospital classification used by the former POG institutions (51). The Evans (CCG) classification emphasized tumor extent, as determined radiographically; in particular, whether the tumor extended beyond the organ or structure of origin and if it crossed the midline. The St. Jude (POG) classification relied on surgicopathologic staging, emphasizing lymph node involvement (Table 36-6). Both staging systems have prognostic value, but differences and discrepancies between the two systems presented obstacles to population studies of neuroblastoma. For example, a tumor that crossed the midline but was completely resected was Evans stage III but St. Jude stage A, whereas a localized tumor with ipsilateral, nonadherent lymph node involvement was Evans stage I but St. Jude stage C.
TABLE 36-5 Diagnosis of Neuroblastoma. | ||
|---|---|---|
|
TABLE 36-6 Tumor Staging Systems for Neuroblastoma. | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree