Neck
Mary E. Fallat
Kristina A. Bryant
Division of Pediatric Surgery, Kosair Children’s Hospital, Department of Pediatrics, University of Louisville, Louisville, Kentucky 40202.
Division of Pediatric Surgery, Kosair Children’s Hospital, Department of Pediatrics, University of Louisville, Louisville, Kentucky 40202.
Although the neck is a small portion of the total body area, it accounts for a relatively large amount of pathology encountered by pediatric surgeons in clinical practice (Table 56-1). The neck is often the focus of abnormalities that require a working knowledge of embryology, anatomy, and clinical diagnosis to effectively formulate a treatment plan. Because the rich lymphatic supply in the neck offers a medium for both benign and malignant disease, good judgment is critical to determine the difference. Diagnosis might involve a formal surgical procedure or a less invasive test, such as fine-needle aspiration (FNA) or a radiographic study. A treatment plan might involve surgery or a conservative approach, depending on the diagnosis. The need for surgery may be complicated by antecedent infection. Few other operative sites require as much skill and judgment to determine the timing of, need for, and anatomic approach to an operation.
EMBRYOLOGY
The pharyngeal primordium relevant to this chapter is the branchial apparatus, consisting initially of five paired endodermal pharyngeal pouches and four corresponding ectodermal branchial clefts with mesodermal branchial arches between consecutive pairs (1).
Of the four branchial clefts visible in the fifth week of gestation, only the most dorsal portion of the first cleft persists as the external auditory canal. The eustachian tube and cavity of the middle ear are formed from the corresponding portion of the first pharyngeal pouch, and the closing plate between pouch and cleft is represented by tympanic membrane. The remaining branchial clefts obliterate during the sixth and seventh weeks. The approximate location of obliterated embryonic clefts, arches, and pouches that may have clinical significance is outlined in Table 56-2, and is relevant to the origin of the epithelium-lined cysts, sinuses, and fistulas encountered in clinical practice.
The thyroid gland is formed by the median thyroid anlage (origin marked by foramen cecum) and the lateral thyroid anlage (the fourth and fifth branchial pouch complex). Elongation of the embryo leaves the thyroid gland caudal to its point of origin. In the fifth week of development, the attenuated stalk (thyroglossal duct) loses its lumen and fragments, leaving a pit at the ultimate location of the foramen cecum and a distal remnant attached to the thyroid isthmus, which often persists as a pyramidal lobe. Mesodermal anlage of the body of the hyoid bone usually appear soon after, and if the thyroglossal duct is not yet obliterated, a persistent thyroglossal duct related to the hyoid bone results.
The thymus develops as bilateral sacculations from the ventral aspects of the third pharyngeal pouches during the sixth week of life. The thymus primordia retain a lumen, the thymopharyngeal duct, which obliterates after medial fusion of its two halves. A cervical thymic cyst is believed to result from arrest of the normal embryonic migration of the thymic primordia, by the persistence of the thymopharyngeal tract, or by cystic degeneration of Hassall corpuscles and other thymic components.
The lymphatic system develops from the mesoderm prior to the parotid gland encapsulation, and after the submandibular and sublingual glands encapsulate. This delayed encapsulation of the parotid gland is critical because it results in the entrapment of the lymph nodes and lymphatic channels within the parenchyma of the parotid gland. Hyperplastic changes or neoplastic involvement of these nodes may manifest as a parotid mass.
TABLE 56-1 Differential Diagnosis of Neck Masses in Children. | |
|---|---|
|
ANATOMY
Surface Anatomy
In the anterior neck, the superior border of the manubrium demarcates the inferior extent of the suprasternal space (jugular notch), limited laterally by the sternal heads of the sternocleidomastoid muscles. In the midline anterior neck, midway between the suprasternal space and the chin, the laryngeal prominence (Adam’s apple) is formed by the V-shaped thyroid cartilage of the larynx. At the superior border of the prominence, the laryngeal notch is palpable. Above the laryngeal prominence, the hyoid bone can be palpated. The chin is formed by the mental protuberance of the mandible, and at the posterior border of the jaw, the prominent angle is continued superiorly as the ramus.
The root of the neck is the junctional area between the neck proper and the thorax. It is limited laterally by the first rib, anteriorly by the manubrium of the sternum, and posteriorly by the first three thoracic vertebrae.
Fasciae of the Neck
The superficial fascia of the head and neck is continuous with the superficial fascia of the pectoral, deltoid, and back regions. The deep fasciae consist of the outer investing layer, the middle cervical layer, the prevertebral, and the pretracheal fasciae. The outer investing layer (external cervical fascia) completely surrounds the neck like a stocking, extending from the clavicle over the mandible to the zygoma. In the anterior triangle, this layer is bound to the hyoid bone and is subdivided into suprahyoid and infrahyoid portions. The middle cervical fascia, composed of two layers, encloses the strap muscles of the neck.
The prevertebral fascia covers the anterior aspect of the cervical vertebrae. A potential cleft between the prevertebral fascia and the fascia of the pharynx, the retropharyngeal space, is limited superiorly by the base of the skull and laterally by the attachment of prevertebral to middle cervical fascia. Inferiorly, the retropharyngeal space communicates with the posterior mediastinum.
The visceral component of the neck is located between the prevertebral and middle cervical fascia. It contains the major arteries and nerves in the neck, the cervical portions of the digestive and respiratory systems, and the thyroid and parathyroid glands. The visceral (pretracheal) fascia is a tubular projection into the neck of the visceral fascia of the mediastinum, where it is continuous with the fibrous pericardium. It encloses the esophagus, trachea, pharynx, and larynx, and contributes laterally to the formation of the carotid sheath.
Lymph Nodes
Lymph nodes that drain lymph from the head and neck are arranged symmetrically on both sides of the neck. Vertical chains of nodes are present along the entire length of the carotid sheath and receive most of the lymphatic drainage from the head and neck. Additional lymphoid tissue pertinent to this chapter is located in the Waldeyer ring, which includes the nasopharynx, tonsils, base of the tongue, and oropharyngeal wall.
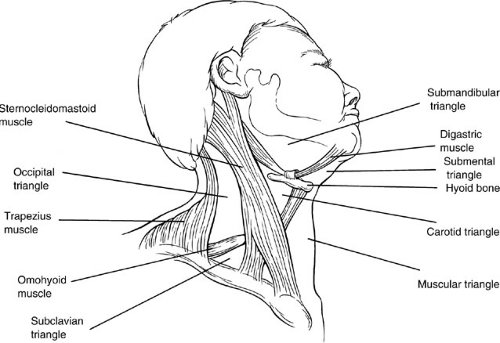
Triangles of the Neck
The neck is divided into three main triangles—the anterior, the posterior, and the suboccipital (Fig. 56-1). The anterior triangle of the neck is bounded posteriorly by the anterior border of the sternocleidomastoid muscle and anteriorly by the midline of the neck. The base is formed by the lower border of the mandible, and the apex is at the
sternum. This triangle can be subdivided into three paired and one common triangle—the muscular, carotid, and submandibular (digastric) triangles, and the unpaired submental triangle. The posterior triangle of the neck is divided by the posterior belly of the omohyoid into a small subclavian and a large occipital triangle. The suboccipital triangle is located in the posterior midline.
sternum. This triangle can be subdivided into three paired and one common triangle—the muscular, carotid, and submandibular (digastric) triangles, and the unpaired submental triangle. The posterior triangle of the neck is divided by the posterior belly of the omohyoid into a small subclavian and a large occipital triangle. The suboccipital triangle is located in the posterior midline.
TABLE 56-2 Relationship of Embryonic Derivatives to Clinical Abnormalities. | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
DIAGNOSTIC APPROACH
The initial evaluation of infants and children with head and neck complaints begins with a complete history and physical examination. Eighty percent of neck masses in children are benign. Important historical points to consider include site and duration of symptoms, systemic signs of illness such as unexplained fever or weight loss, associated illnesses such as otitis media or streptococcal pharyngitis, and exacerbating or ameliorating factors. An inflammatory lesion usually has a short duration or may recur. A lesion of long duration, or one identified at or soon after birth, is more likely to be congenital, benign, or both. A painless, rapidly enlarging mass is often malignant. The initial symptoms of a solid tumor in the head and neck area are often nonspecific and caused by the effect of the mass on surrounding tissues.
Physical examination of the child includes careful inspection and palpation of the neck abnormality, as well as examination of the face, scalp, ears, and oral cavity, which may yield the initiating source of the neck pathology. A general physical examination, including the chest, abdomen, genitalia, and extremities, completes the evaluation. Subsequent diagnostic workup is guided by the preliminary history and physical examination.
In some situations, such as a case of presumably benign lymphadenopathy, the surgical consultant may decide that no workup is necessary. A simple complete blood count with a peripheral smear may help define an acute infection. If the diagnosis is questionable, an ultrasound, computed tomography (CT) scan, magnetic resonance imaging (MRI), or FNA often guides the need for therapeutic surgical intervention.
Radiographic Studies
Ultrasound is particularly helpful in distinguishing solid from cystic masses or in determining maturation of a deep cervical abscess. Doppler ultrasound can distinguish a vascular mass, such as a hemangioma, from an avascular lesion. A CT scan is more useful when a tumor is diagnosed or suspected, and this modality also helps delineate depth of, anatomic detail, or thoracic extensions of neck masses. MRI provides three-dimensional anatomic detail, but has drawbacks including high cost and the need to sedate younger patients to prevent motion artifact. It is particularly useful if spinal cord or intracranial extension of a tumor is suspected. T2-weighted imaging identifies areas of increased vascularity, and T1-weighted images enhanced with gadolinium allow precise localization of central nervous system tumors. A good quality T2-weighted image can also delineate the position of the facial nerve with respect to a parotid tumor.
Gallium 67 scanning is useful in the initial staging and monitoring of disease response or recurrence in high-grade non-Hodgkin’s lymphomas. Positron emission tomography (PET) is a diagnostic imaging technique that maps the location and concentration of radionuclide-labeled tracers (2). In contrast to CT and MRI, which are modalities primarily depicting anatomic details, PET provides information about metabolism. Neoplastic cells incorporate more of the glucose analog and have more radio-intense images than surrounding normal tissues.
Additional radiographic studies that may be needed to evaluate a neck mass include chest radiograph, bone scan, and CT scans of the abdomen, chest, or brain as part of a metastatic workup if a malignant tumor is suspected or diagnosed. A chest X-ray can rule out pulmonary involvement in tuberculosis or a potentially systemic fungal disease.
Fine-Needle Aspiration
The advantages of FNA over excisional biopsy include the ability to perform the procedure in an office or clinic setting, no need for general anesthesia, lower cost than open biopsy, diagnosis within 24 hours, and no subsequent scar. Enough material can usually be generated for cytology and culture, although a sufficient sample is needed for flow cytometry, chromosome analysis, or electron microscopy, and this may be a limiting factor for FNA. A lymph node more than 1 cm in diameter that persists or grows for longer than 6 weeks and does not respond to antibiotics should be considered for biopsy by FNA.
The information obtained on FNA can be either reassuring as benign to the child’s physician and child’s parents or can lead to more rapid treatment for another defined entity. Because of the possibility of sampling error, excisional biopsy should be considered if a lymph node or neck mass continues to enlarge over 2 to 3 weeks or persists in size for 3 months after FNA.
BENIGN LESIONS
Lymphadenopathy
Benign cervical lymphadenopathy accounts for most neck masses in children, the causes of which are protean. Although viral and bacterial etiologies are most common, atypical mycobacteria infections, mononucleosis, and cat-scratch disease (CSD) are other frequent diagnoses. Tuberculosis and fungal diseases are occasionally encountered.
If the underlying cause is evident, treatment is usually directed toward the etiologic agent or watchful waiting is advocated. Under these circumstances, the lymphadenopathy often regresses, although frequently, it does not totally disappear.
If the underlying cause is evident, treatment is usually directed toward the etiologic agent or watchful waiting is advocated. Under these circumstances, the lymphadenopathy often regresses, although frequently, it does not totally disappear.
Viral and Bacterial Etiologies
The pediatric surgeon is often asked to evaluate a child with long-standing cervical lymphadenopathy. Careful questioning may reveal a history of frequent upper respiratory infections, otitis media, tonsillitis, sinusitis, or allergies associated with rhinitis. The lymphadenopathy may fluctuate in size, depending on whether the child has an active infection. The nodes are usually mobile and located in the upper anterior cervical areas. They may be bilateral and slightly tender in the presence of active infection. In most cases, this adenopathy can be treated conservatively, and an associated identified bacterial infection is treated with antimicrobial agents.
If biopsy seems indicated, an FNA or biopsy can be done and usually reveals reactive lymphadenopathy, allowing continued observation and alleviating parental anxiety. An aspirate can also be obtained for culture, although culture proves positive in only about 15% of cases (3).
Bacterial lymphadenitis most typically involves Staphylococcus aureus or group A β-hemolytic streptococcal etiologies. Bacterial infections may also be characterized by rapid nodal enlargement followed by slow evolution to an abscess or suppurative lymphadenitis with overlying skin erythema and fluctuance. Young children tend to have associated fever until the abscess matures and can be drained, despite appropriate antimicrobial therapy. Ultrasound may help to plan optimal timing of the surgical drainage.
The timing and approach to surgical therapy of a probable phlegmon or abscess is dictated by suspected etiology and the need for diagnosis versus therapy. The vast majority of neck abscesses should be drained under general anesthesia in the operating room. This allows for adequate drainage, identification of any loculations, ensures control of the airway, and allows for control of any bleeding. A transverse incision is made over the main area of fluctuance unless this is inappropriate for cosmetic reasons, and it should be long enough to enable the surgeon to probe the abscess cavity and break up any loculations. This ensures adequate drainage and allows placement of a passive drain, such as a Penrose, or packing material. Incisions made very high or very low in the neck should be made parallel to the mandible or clavicle, respectively, and at least 1 or 1.5 cm away from these bony landmarks to preclude significant scarring.
Adequate surgical drainage in combination with appropriate antimicrobial therapy usually resolves simple suppurative processes. Identification of an unusual organism or abscess recurrence may indicate an underlying immunosuppressive illness or congenital anomaly and should prompt an appropriate workup.
Tuberculosis, Atypical Mycobacteria, and Fungal Etiologies
The oropharynx and respiratory tract act as the reservoir for these infections because atypical mycobacteria (AMB) can also be cultured from these sites in asymptomatic patients. The most common organisms causing infection in humans are Mycobacterium avium, Mycobacterium intracellularis, and Mycobacterium scrofulaceum. The collective group is now known as the M. avium complex (MAC). Unlike Mycobacterium tuberculosis (TB), person-to-person transmission of MAC has not been documented (4,5).
Uncomplicated cases of AMB often present as a group of upper cervical nodes, which are initially rubbery, firm, and nontender, located close to the mandible. They later become matted together to form a confluent mass. The child is usually otherwise well, whereas cases of TB are more common in immunocompromised children and are accompanied by symptoms or signs of illness, such as fever and respiratory complaints. An occasional patient has a superimposed pyogenic infection with fever, and an acid-fast stain and culture for mycobacteria at the time of abscess drainage may prove helpful in diagnosis if the wound later fails to heal properly.
An intradermal skin test with standard tuberculin antigens may be helpful because there is a cross-reactivity between AMB and TB. Reactions to intermediate-strength purified protein derivative (5 tuberculin units) are usually 5 to 10 mm in diameter in cases of AMB, and significantly greater than 10 mm in TB cases. FNA or incisional biopsy in cases of both AMB and TB is diagnostic.
If tuberculosis is strongly suspected, a trial of antituberculous therapy may be indicated pending culture results. Atypical mycobacteria infections generally respond poorly to chemotherapy. Incision and drainage or incomplete nodal excision of AMB invariably leads to recurrence or cutaneous draining sinuses. Complete excision is ordinarily curative, although there may be a role for adjunctive antimicrobial therapy with clarithromycin and rifabutin (6,7,8,9).
Fungal infections are less frequent and more often seen in immunocompromised patients. The most frequently reported pathogen is Candida albicans.
Mononucleosis (Epstein-Barr Virus Infection)
The lymphadenopathy associated with mononucleosis is usually bilateral and more common in the adolescent than the child. Hepatosplenomegaly may be present, and a heterophil antibody test (monospot) or Epstein-Barr virus (EBV) titers are positive. Young children with acute EBV infection are likely to be heterophil antibody negative, although this rapid test may be useful in the older
child or adolescent. Cytomegalovirus (CMV) can also cause mononucleosis with associated adenopathy. Up to 75% of patients with CMV infections will have cervical adenopathy. The lymphadenopathy resolves within weeks.
child or adolescent. Cytomegalovirus (CMV) can also cause mononucleosis with associated adenopathy. Up to 75% of patients with CMV infections will have cervical adenopathy. The lymphadenopathy resolves within weeks.
An occasional patient with a history of mononucleosis later develops Hodgkin’s disease or non-Hodgkin’s lymphoma, but the association is not common enough to warrant routine node biopsy in every patient with mononucleosis. Cervical node biopsy is warranted in patients who have persistent or recurrent lymphadenopathy in the face of normal or declining EBV titers following a documented case of mononucleosis.
Cat-Scratch Disease
The classic features of CSD include self-limited, regional lymphadenopathy occurring in healthy people following a cat scratch or bite distal to the affected node. Common sites of node enlargement other than the neck are the axilla, elbow, and groin (10). Other animals, such as dogs and monkeys, have also been implicated in transmission (11).
Bartonella henselae, a member of the Rickettsiaceae family, is the etiologic agent (10,12). A B. henselae-based serologic indirect fluorescent-antibody test appears to be both a sensitive and specific diagnostic tool. Enzyme immunoassays for detection of antibodies to B. henselae have also been used. Tissue can be tested by polymerase chain reaction. The domestic cat is a major persistent reservoir for the organism, and the cat flea (Ctenocephalides felis) is a vector.
Most children have mild symptoms and a benign clinical course. Severe complications, including encephalitis, follicular conjunctivitis, and neuroretinitis, have also been described. A papule may form at the site of primary inoculation. In most cases, the disease resolves spontaneously within a few months. Lymph node biopsy reveals multiple microabscesses or granulomas. Occasionally, nodes suppurate and require drainage. Antibiotics with activity against Bartonella include azithromycin, gentamicin, rifampin, trimethoprim-sulfamethoxazole, and ciprofloxacin (13).
Other Infectious Causes of Lymphadenitis
Other causes of cervical lymphadenitis include actinomyces, toxoplasmosis, tularemia, Yersinia pestis, Nocardia species, Mycoplasma pneumoniae, and Pasturella multocida (after an animal bite or scratch). Viral causes include adenovirus, enterovirus roseola (herpesvirus 6), rubella, and Parvovirus B 19 (the latter can cause bilateral cervical and intraparotid adenopathy).
Other Noninfectious Causes of Lymphadenopathy
There are a few more unusual or rare noninfectious causes of lymphadenopathy, including Kawasaki and Kikuchi disease, sarcoidosis, Rosai-Dorfman (sinus histiocytosis with massive lymphadenopathy), and histiocytosis X.
Surgical Management of Lymphadenitis
In cases of cervical lymphadenitis that do not respond to a course of standard antimicrobial therapy or TB therapy, or lymphadenitis that is persistent or enlarges after diagnosis of mononucleosis or CSD, surgical excision for diagnosis is appropriate. In cases of cervical lymphadenitis that fit the diagnosis of AMB infection clinically, surgical excision without drainage is the recommended treatment (4). Definitive surgery in the latter circumstance should involve complete excision of all involved sinus tracts and nodes, if feasible. Cases of parotid involvement that are treated with superficial parotidectomy, or that have neural involvement that precludes complete resection of involved nodes, usually heal normally (5,9). Adjunctive antimicrobial therapy for cases of AMB or CSD should probably be reserved for disseminated or problematic disease.
Enlarged cervical nodes may require biopsy in almost any site in the neck. A small transverse incision made in a skin crease overlying the node is usually sufficient for biopsy. Nodes in the posterior triangle are usually superficial and easily excised, with careful avoidance of the spinal accessory nerve. In most cases, nodal tissue can be directly approached and then teased away from surrounding tissue using blunt dissection to avoid damage to important structures.
To approach the supraclavicular area, an incision is made over the lateral edge of the sternocleidomastoid muscle. After incising the platysma, the nodal tissue between the omohyoid muscle superiorly, external jugular vein laterally, carotid sheath medially, and clavicle inferiorly are dissected free. The anterior scalene muscle, brachial plexus, and phrenic nerve can be visualized at the base of the dissection. Submandibular node excision requires careful avoidance of the mandibular branch of cranial nerve VII.
INFLAMMATORY PROCESSES AND SERIOUS INFECTIONS
Salivary Glands
Sialadenitis, or inflammation of a salivary gland, is one of the most common benign entities affecting children and can usually be distinguished from neoplasia by the presence of episodic pain and swelling (14). The process may be due to a viral infection, a bacterial suppurative infection, a chronic process without determined cause, or a granulomatous process. It is more common in boys, and the parotid gland is the salivary gland most often involved. Acute suppurative sialadenitis is usually a disease of infants due to S. aureus and may have associated bacteremia.
The process may progress to abscess formation, requiring incision and drainage that is curative in conjunction with antibiotics.
The process may progress to abscess formation, requiring incision and drainage that is curative in conjunction with antibiotics.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree