Lymphatic and Venous Vascular Malformations
Anne C. Fischer
Sally E. Mitchell
Anthony P. Tufaro
Department of Surgery, Division of Pediatric Surgery, Johns Hopkins University, Johns Hopkins Hospital, Baltimore, Maryland 21287.
Interventional Pediatrics Associates, Inter- ventional Radiology, Johns Hopkins Medical Institutions, Baltimore, Maryland 21287.
Department of Surgery, Division of Plastic and Reconstructive Surgery, Johns Hopkins University, Johns Hopkins Hospital, Baltimore, Maryland 21287.
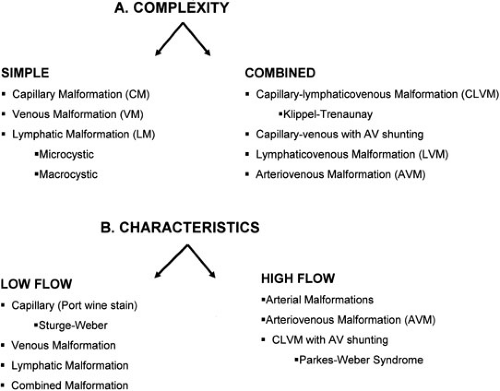
Vascular malformations are among the most common birth defects and are the sequelae of congenital errors of morphogenesis. These lesions can present with a diverse spectrum of clinical signs and symptoms and in a variety of anatomic locations. Any individual component from the normal channels of blood and lymphatic flow can constitute the basis of vascular malformations. These anomalous channel types can include aberrancies in arteries, capillaries, veins, and lymphatics. The developmental abnormalities of veins and lymphatics are the focus of this chapter. Due to the varied clinical manifestations of vascular malformations, the complexity of this specialty area mandates a clinical expertise and an orchestrated multidisciplinary approach that has heretofore been underappreciated. These vascular malformations can be characterized by the complexity of the lesions or the inherent flow characteristics (Fig. 103-1).
LYMPHATIC SYSTEM
The actual incidence of lymphatic malformations is unknown, but it is believed to exceed the original estimate of 6.3% of all malformations (1). These malformations are seen in any region rich with lymphatics that were sites of early lymphatic ontogeny. The most common anatomic locations are cervicofacial (75%) and axillary (20%), followed by mediastinal and retroperitoneal. An understanding of lymphatic embryology creates an appreciation of the predilection of postnatal lymphatic malformations to lymphatic-rich areas in ontogeny. The lymphatic system develops from six primitive endothelial sacs that first appear as jugular sacs by the second month in utero (2,3). These six lymphatic-rich regions include two paired jugular sacs in the neck, an endothelial sac at the root of the mesentery, a sac at the cisterna chyli, and two paired posterior inguinal sacs. Secondary endothelial channels interconnect the bilateral jugular sacs, crossing the neck, mediastinum, and abdomen. The thoracic duct longitudinally connects the cisterna chyli with the cervical jugular lymphatic sacs that empty into the left subclavian vein. The retroperitoneal sac at the root of the mesentery is the origin of the mesenteric lymphatics; likewise, the bilateral inguinal sacs propagate to form to the lower-extremity lymphatics. All primitive lymphatic sacs are highly proliferative; thus, lymphatic malformations are believed to result from developmental arrest as the vascular channel propagates or becomes disconnected and sequestered. The etiology, then, is a fundamental defect in the development of lymphatic channels with absent (aplastic), insufficient (hypoplastic), or obstructed (ectatic) efferent pathways. Abnormal lymphatic morphogenesis results in altered tissue architecture and subsequent altered lymphatic flow.
Solid endothelial lymphatic channels parallel the development of embryonic blood vessels and arborize to constitute the peripheral lymphatic system. These solid channels become patent vessels that support the circulation of lymphoblasts. The lymphatic system is the primary conduit to recirculate lymphocytes from interstitial tissues back into the bloodstream. Circulating lymphocytes are returned to the bloodstream, and macromolecules, including fat, are systemically absorbed through the lymphatics. The lymphatic system reflects not only the interstitial composition, but also the interstitial fluid volume. Increased interstitial fluid volume results in enhanced lymphatic flow.
Antegrade lymphatic flow is dependent on intramuscular contractions and intrathoracic pressure because valves, to resist retrograde flow, exist only within the deep lymphatic system; all are essential components to return lymph to the central venous system.
Antegrade lymphatic flow is dependent on intramuscular contractions and intrathoracic pressure because valves, to resist retrograde flow, exist only within the deep lymphatic system; all are essential components to return lymph to the central venous system.
Lymphatic Malformations
Typically referred to as lymphangiomas, lymphatic anomalies are more precisely designated as malformations because the appendix “-oma” inherently implies the hyperplastic and proliferative characteristics of tumors (4,5). In contrast, these lesions are not associated with unabated cellular proliferation or hyperplastic growth. An increase in size is not related to independent cellular growth, but rather to enhanced accumulation of fluid or recruitment of additional lymphatics. Combined vascular anomalies can coexist in the context of abnormal lymphatic tissue and frequently are lymphaticovenous or capillary-lymphatic malformations.
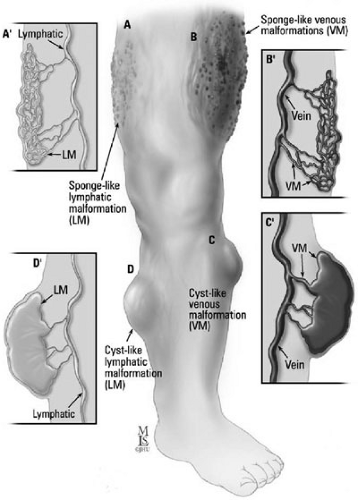
A layer of flattened endothelial cells lining each cyst is characteristic of the histology of lymphatic malformations. Fibrous or inflammatory changes extrinsic to the endothelium are included within the cyst. The gross histology varies from a large unilocular cyst, such as a macrocystic structure, to multiple small cysts, such as a microcystic formation (Figs. 103-2A and 103-2D). These lesions can be localized or extensive, infiltrating local tissue architecture. Lymphatic malformations fluctuate in size secondary to trauma or infection, and undergo fibrotic shrinkage only after recurrent episodes of cellulitis and lymphangitis.
Those malformations that permeate through the epidermis as superficial vesicular lesions are the most vulnerable to repetitive cellulitic infections. Such superficial vesicular lesions are secondary to a deeper lymphangiomatous component involving the dermis. To avoid rapidly progressive lymphangitis, antibiotic therapy should be initiated expeditiously and directed at streptococcal and staphylococcal organisms. Perineal locations require concomitant gram-negative enteric coverage. Recurrent episodes of infection lead to fibrotic shrinkage of the lymphangioma.
Those malformations that permeate through the epidermis as superficial vesicular lesions are the most vulnerable to repetitive cellulitic infections. Such superficial vesicular lesions are secondary to a deeper lymphangiomatous component involving the dermis. To avoid rapidly progressive lymphangitis, antibiotic therapy should be initiated expeditiously and directed at streptococcal and staphylococcal organisms. Perineal locations require concomitant gram-negative enteric coverage. Recurrent episodes of infection lead to fibrotic shrinkage of the lymphangioma.
Diagnosis
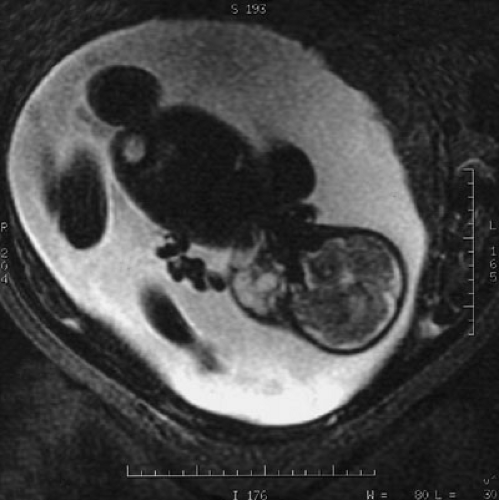
Lymphatic malformations detected very early prenatally at the end of the first trimester manifest diffently than those discovered postnatally (6,7). Those fetal lymphatic malformations diagnosed prior to 30 weeks’ gestation, that usually arise in the posterior cervical region are associated with fetal hydrops and abnormal karyotypes. The abnormal karyotypes typically include Turner’s syndrome, but the trisomies (13,18,21,11q/22Q, and a 22 mosaic) have also been reported; therefore, an amniocentesis is highly recommended. Noonan’s syndrome and fetal alcohol syndrome have also been associated with prenatally diagnosed lymphatic malformations. Spontaneous resolution of these malformations has been documented. In contrast, persistence of the malformation with subsequent fetal hydrops portends a poor outcome.
The majority of lymphatic malformations are detected postnatally and are clinically evident early; more than 65% of diagnoses are determined at birth and more than 90% by the age of 2 years (8). Overall, they are unusual lesions without racial or sexual predilection. Radiographic imaging may necessitate several modalities, depending on the anatomic complexity and potential extension or field effect of the anomaly. Prenatal lesions can be detected by duplex ultrasound (US) and are well-imaged in the facial region with three-dimensional (3D) ultrasonography. Advances in 3D prenatal imaging have permitted earlier detection of cervicofacial malformations that may require prenatal planning for an ex utero intrapartum procedure to prevent emergent loss of the airway from anatomic distortion and an obstructing mass effect (Fig. 103-3). Superficial lesions mandate a high-frequency US with color flow duplex as the initial least invasive study. However, magnetic resonance imaging (MRI) with T2-weighted images is ideal in identifying a malformation as lymphatic and defining the extent of the malformation, particularly in complex anatomic regions such as the head and neck and mediastinum (9,10). Magnetic resonance venography (MRI/MRV) is the best modality to image lymphangiomatous involvement of the peritoneum and contiguous retroperitoneum. In conjunction with 3D imaging, radionucleotide lymphoscintigraphy and dynamic lymphangiography are powerful modalities to trace lymphatic flow and delineate the precise anatomic point of obstruction or abnormality.
Treatment
Surgical resection with complete excision for a given anatomic region is performed whenever technically possible. Although these lesions are benign and have a demarcated plane of dissection, they do not stay confined to normal tissue planes and can envelop contiguous structures. Therefore, the paradigm is to first identify the anatomic regions involved, and then to stage the surgery, excising with meticulous dissection as much of the malformation as possible in a given anatomic region without damaging normal vital structures. For instance, cervicofacial lymphatic malformations can encase major cranial nerves, vessels, and parotid glands; however, they do not mandate a radical extirpation with subsequent loss of function because these are not malignancies. An incomplete debulking dissection near vital structures ensures a high incidence of recurrence and suboptimal outcome, for which a second operation in a reoperative field is not without significant morbidities. Thus, the best opportunity for resectability is the first excision. With extensive lesions, anatomic regions should be defined and staged such that a complete anatomic region is maximally cleared in one setting. If a particular region is subjected to staged resection, then the remaining contiguous lesions may expand
significantly postoperatively; creating a temporary field effect. Preoperative planning for airway compromise, respiratory difficulties, or diaphragmatic paralysis cannot be overemphasized. The incidence of postoperative complications can be 30% or higher, including recurrences and neurologic defects (11). Recurrences range from 10% to 27% following complete resections and up to 50% or higher following debulking or partial resections. Drainage from surgically placed drains is expected for a protracted period of time from weeks to months. Postoperative infections should mandate immediate antibiotic therapy. An advantage of recurrent cellulitis or lymphangitis may be eventual sclerosis of the leaking lymphatics and a dramatic drop in drainage. Other temporary interventions, such as needle aspirations, fail due to rapid lymphatic reaccumulation, but may be useful as a temporizing measure of emergent decompression if airway compromise is imminent.
significantly postoperatively; creating a temporary field effect. Preoperative planning for airway compromise, respiratory difficulties, or diaphragmatic paralysis cannot be overemphasized. The incidence of postoperative complications can be 30% or higher, including recurrences and neurologic defects (11). Recurrences range from 10% to 27% following complete resections and up to 50% or higher following debulking or partial resections. Drainage from surgically placed drains is expected for a protracted period of time from weeks to months. Postoperative infections should mandate immediate antibiotic therapy. An advantage of recurrent cellulitis or lymphangitis may be eventual sclerosis of the leaking lymphatics and a dramatic drop in drainage. Other temporary interventions, such as needle aspirations, fail due to rapid lymphatic reaccumulation, but may be useful as a temporizing measure of emergent decompression if airway compromise is imminent.
Sclerotherapy to reduce the size of the lesion is another strategy that has been used to minimize the extent of surgery or to avoid surgery completely. The mechanism of action of sclerosants is to induce an inflammatory reaction and subsequent fibrosis with endothelial cell destruction and collapse of the cyst. Sclerosants currently available include ethanol, bleomycin, doxycycline, OK-432, and fibrin glue. Macrocystic lesions are easily accessed using US guidance and fluoroscopic control, and are therefore amenable to intralesional injection of sclerosants. Microcystic lesions are less accessible and usually respond less well than macrocystic lesions to sclerotherapy. However, sclerotherapy is possible, although difficult, with microcystic lesions due to the technical difficulty of accessing percutaneously the multiplicity of tiny lymphatic spaces that are not necessarily in continuity. Sclerotherapy usually requires a series of treatments 6 weeks apart to treat the multiple cystic areas adequately.
Ethanol has been widely used for lymphatic and venous sclerotherapy, and its mechanism of action is not only an inflammatory reaction directed to the endothelium, but also induction of endothelial cell necrosis and destruction. Because of its more potent endothelial damage, ethanol has been the sclerosant of choice in renal cysts, hepatic cysts, lymphoceles, and both venous and lymphatic malformations. Absolute ethanol at a total dose of 0.5 to 1 cc per kg of body weight is the dose for directed intralesional sclerotherapy.
Microspheres of oil-bleomycin lipid emulsions (BLM 9 mg per mL) are a more effective sclerosant than bleomycin alone due to the rapid uptake of the lipophilic emulsion in lymphatics and a higher concentration at the intended site of action (12,13). Injection of 0.3 to 0.6 mg per kg intralesionally after cyst aspiration is the recommended approach. Contraindicated in infants younger than 6 months of age, the drug induces transient swelling for up to 2 weeks post therapy and has a myriad of side effects, including fever, diarrhea, vomiting, vesiculation, and scarring.
There is a more limited experience with percutaneous doxycycline, which can be delivered after either needle aspiration or via fluoroscopic or computed tomography (CT)-directed pigtail catheters. The dose ranges from 5 to 20 mg per mL of a diluted 1:4 mixture with a radiopaque diluent of iodohexol for concurrent fluoroscopic imaging. Pain control, fever, and cellulitis are all side effects (14).
OK-432 (Picibanil) is sclerosant developed in Japan that is a lyophilized mixture of group A streptococcus pyogens with penicillin G potassium (15,16). The mechanism of action is an immune-mediated reaction within the cyst, resulting in fibrosis and endothelial destruction. The recommended dose is an intracystic injection of 0.1 mg in 10 mL of saline (0.9% wt per vol) after initial aspiration of the cyst with a total volume not to exceed 20 mL. Repeated injections can be given every 4 to 6 weeks. Fever, induration, erythema, and swelling can last 3 to 7 days. A prospective randomized trial demonstrated that 86% of patients with macrocystic lymphangiomas responded with greater than 60% reduction in cyst size after four injections (17). OK-432 has been subjected to rigorous clinical trials, but is not available currently outside trials in the United States.
All sclerosants induce an inflammatory reaction and edema; thus, all can induce airway compromise if there is resultant edema adjacent to the airway in the cervicofacial region or mediastinum. This approach is typically used in anatomic locations when complete resection is not safe or feasible. The anatomic site and the size of the lesion determine the type of anesthesia, length of intubation, and intensive care monitoring. Laser therapy, using a CO2 laser, is the modality for lymphatic malformations intrinsic to the larynx and airway. Likewise, the laser can treat residual superficial epidermal lymphatic vesicles following resection of the underlying lymphatic malformation. Radiation therapy is no longer considered an acceptable approach because of significant morbidities and complications.
Lymphatic malformations in certain anatomic regions require meticulous planning (Fig. 103-4). Giant cervicofacial lymphatic malformations involve multiple structures, including the tongue, larynx, and parotids, often with bilateral and mediastinal extension. These giant lesions with mediastinal extension are the most challenging of surgical resections; likewise, they have the potential for rapid enlargement and airway compromise with a potential need for earlier intervention and probable tracheostomy. Long-term craniofacial problems from large cervicofacial lymphangiomas can result from maxillofacial hypertrophy and mandibular hypertrophy with malocclusion. Hypertrophy of either the mandible or maxilla induces hypertrophy of the other, and may require multiple osteotomies and ostectomies
to control growth and normalize occlusion. Final outcomes are often disappointing and frustrating to both the patient and the physician. Macroglossia is also not uncommon. These lesions are often microcystic, difficult to sclerose, and difficult to manage. Macroglossia may require several interventional modalities, including preoperative sclerosis and subsequent limited surgical resections to preserve oral function; both modalities can potentially impact lingual nerve function.
to control growth and normalize occlusion. Final outcomes are often disappointing and frustrating to both the patient and the physician. Macroglossia is also not uncommon. These lesions are often microcystic, difficult to sclerose, and difficult to manage. Macroglossia may require several interventional modalities, including preoperative sclerosis and subsequent limited surgical resections to preserve oral function; both modalities can potentially impact lingual nerve function.
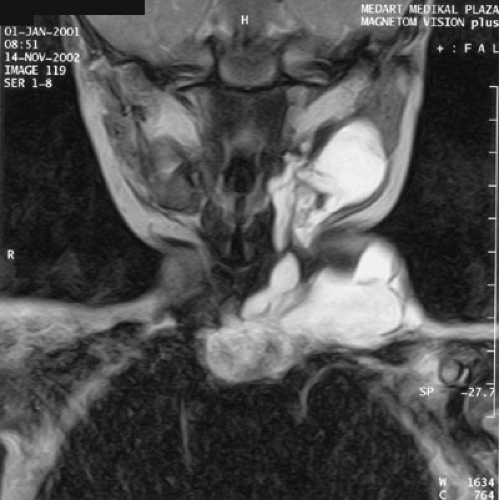 FIGURE 103-4. Coronal T2 magnetic resonance image of a cervicofacial lymphaticovenous malformation with mediastinal extension. |
Lesions of the parotid and cervical area are challenging due to the potential for devastating cranial nerve injuries. Parotid involvement is best managed initially with observation in an effort to avoid surgery in an infant at a time when the facial nerve is difficult to visualize. Planned excision of the lesion, including a superficial parotidectomy avoiding injury to the facial nerve, is done after growth beyond infancy. The hypoglossal nerve is at risk with cervical lesions deep to the digastric muscle in the submandibular area (level I) and near the carotid bifurcation. Injuries result in lingual atrophy and problems with phonation and deglutination. The marginal mandibular branch to the depressors of the labial commissure lies deep to the platysma in the area of the submandibular gland. Injury will cause facial asymmetry and drooling. The facial nerve is at risk in the area of the parotid and superior cervical region. Injuries are truly devastating, leading to facial asymmetry, problems with phonation and feeding, and loss of periorbital musculature with an inability to blink and protect the cornea. Proximal facial nerve injuries are best managed by immediate microsurgical repair with interrupted monofilament suture, and distal injuries are often not amenable to repair.
Thoracic Duct Anomalies: Chylothorax
A chylothorax results from leakage of lymphatic fluid into the thoracic cavity, typically presenting as a right pleural effusion. In 60% to 70% of neonates, the thoracic duct arises from the cisterna chyli, ascends on the right until the fourth to sixth vertebrae, and then crosses the midline to ascend along the left to empty into the left subclavian vein. A congenital chylothorax is the most common cause of pleural effusions in the fetus and neonate, and is usually caused by a malformation involving the thoracic duct (18). Anomalies associated with a congenital chylothorax include trisomy 21, Turner’s and Noonan’s syndromes, pulmonary lymphangectasia, extralobar pulmonary sequestration, and esophageal atresia. Among infants and children, the most common etiology of a chylous thorax is secondary to trauma, including iatrogenic trauma such as cardiothoracic or intrathoracic procedures (coarctation of the aorta, Blalock-Taussig shunts, and Glenn procedures), birth trauma, and blunt thoracic or spinal injury. If effusions are bilateral, then underlying venous hypertension secondary to a central venous thrombosis, such as a superior vena cava obstruction, may be the etiology. Fontan procedures are associated with venous hypertension and chylothoraces may result. It cannot be overemphasized that investigation for systemic venous hypertension causing a refractory chylous thorax be done. This mandates diagnostic and therapeutic measures first to reduce the venous hypertension.
A pleural effusion is diagnostic for chyle if a cell count exceeds 60% lymphocytes or if there is microscopic detection of chylomicrons by Sudan red staining. Daily loss of chyle leads to hypoalbuminemia, hyponatremia, lymphopenia, marked weight loss, and immune dysfunction. Chyle has a milky appearance in the presence of enteral intake, but can appear serous if the patient is either not eating or ingesting a diet restricted to medium-chain triglycerides (MCT). Of note, milky effusions can result from etiologies other than chyle. Potential explanations include intrapleural hyperalimentation or inflammatory conditions associated with cholesterol pleural effusions, such as tuberculosis, filiaris, and rheumatoid arthritis.
Once the effusion is diagnosed as chyle, initial treatment consists of nonoperative management with a thoracostomy tube for drainage and either an elemental diet with MCT (Portagen) or nutrition restricted to total parenteral nutrition. If the effusion is of traumatic etiology and persistent, then enteral intake should be totally prohibited. Chyle loss may be 1,500 mL per day per adult or
100 mL per year of age per day for children (19). Most chylous effusions resolve, but persistent or unmanageable chyle losses refractory to nonoperative management mandate intervention. Administration of the somatostatin analogue, octreotide, (1 to 4 mcg per kg per hour) has demonstrated efficacy in some reports, but not all patients respond and its use in children is a point of uncertainty. Videoscopic thoracic duct ligation has become a standard surgical intervention (20). Pleuroperitoneal shunts have been successfully used in atraumatic chylothoraces (21) and following cardiac surgery (22). For precise imaging, nuclear medicine lymphoscintigraphy can identify the region of lymphatic disruption and a lymphangiogram can reveal anatomic detail. Percutaneous embolization of a leak in the thoracic duct using fluoroscopic guidance is a more recent option, using standard embolization techniques (23). If the site of leak is difficult to visualize intraoperatively or fluoroscopically, preoperative enteral cream administration increases lymphatic flow and simplifies visualization. We have successfully performed lymphatic-venous microsurgical repairs for excision of malformations or repair of the thoracic duct.
100 mL per year of age per day for children (19). Most chylous effusions resolve, but persistent or unmanageable chyle losses refractory to nonoperative management mandate intervention. Administration of the somatostatin analogue, octreotide, (1 to 4 mcg per kg per hour) has demonstrated efficacy in some reports, but not all patients respond and its use in children is a point of uncertainty. Videoscopic thoracic duct ligation has become a standard surgical intervention (20). Pleuroperitoneal shunts have been successfully used in atraumatic chylothoraces (21) and following cardiac surgery (22). For precise imaging, nuclear medicine lymphoscintigraphy can identify the region of lymphatic disruption and a lymphangiogram can reveal anatomic detail. Percutaneous embolization of a leak in the thoracic duct using fluoroscopic guidance is a more recent option, using standard embolization techniques (23). If the site of leak is difficult to visualize intraoperatively or fluoroscopically, preoperative enteral cream administration increases lymphatic flow and simplifies visualization. We have successfully performed lymphatic-venous microsurgical repairs for excision of malformations or repair of the thoracic duct.
Intraabdominal Lymphatic Malformations
Lymphatic cysts in the abdominal or retroperitoneal region are believed to be developmental sequestrations of the embryonic lymphatic sacs that continue to grow independently as separate islands of lymphatic tissue. These cysts can be in the omentum, mesentery, or retroperitoneum. Lymphatic cysts are classically lined by an endothelial cell layer; those with a differing histology, such as mesothelial or columnar cells, may reflect an embryologic origin derived from peritoneal or mesonephric remnants, respectively. Most children present either without symptoms or with a palpable mass; however, abdominal distension and intestinal obstruction can occur. Preoperative imaging by US and CT with intravenous (IV) and oral contrast is often the best modality, although MRI may be helpful in some circumstances. Complete surgical excision may necessitate a limited intestinal resection. Extensive mesenteric and retroperitoneal involvement may require a more extensive resection with marsupialization of the residual portions of the malformation. Intraoperative argon beam therapy has been reported for a nonresectable lesion (24). Percutaneous sclerosis is another potential option. Long-term follow-up is mandated for detection of recurrence or the presence of chylous ascites in those with extensive involvement.
Lymphangiectasia
Abnormal lymphatic development when identified in one organ system often coexists in multiple organ systems, representing a characteristic multiorgan pattern of abnormal lymphatic development. Such systemic lymphangiomatosis is characterized by multicentric lymphatic malformations. Common clusters include liver and spleen lymphangiectasias, as well as lung, bone, and spleen involvement. Often, plain radiographs incidentally reveal severe osteolytic and sclerotic bony changes which mandate systemic evaluation that then identifies multi organ involvement. No systemic therapy is available, and a poor prognosis is evident in refractory cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree