Kidney
Michael A. Keating
Mark A. Rich
Department of Surgery, Division of Urology, University of South Florida School of Medicine, Nemours Children’s Clinic, Orlando, Florida 32806.
Department of Surgery, Division of Urology, University of South Florida School of Medicine, Nemours Children’s Clinic, Orlando, Florida 32806.
INTRODUCTION
The paired kidneys are crucial to the well-being of every child. As the organs of hematologic cleansing and urinary excretion, they play a central role in fluid, electrolyte, and acid–base balance. In addition, they also provide endocrine function, with known roles in vitamin D metabolism and the production of erythropoietin and renin.
ANATOMY
Familiarity with renal anatomy establishes the basis for understanding and managing many diseases of the kidney, regardless of whether they are surgical, medical, traumatic, or neoplastic.
Gross Anatomy
Location, Size, and Orientation
The kidneys of children share many characteristics with those of adults, although certain differences having clinical implications exist. Positioned slightly above the level of the umbilicus and located on either side of the vertebral column, these paired, bean-shaped organs are well protected in the retroperitoneum. The right kidney usually is more dependently positioned as a consequence of its displacement by the liver. Medially, the organs are buttressed by the paraspinous muscles. Posteriorly, their upper poles are shielded by the lower ribs. However, the underdeveloped abdominal musculature of children and their softer ribs offer the anterolateral surface less effective protection against trauma. Here, an intimate association with several adjacent organs readily explains the high incidence of associated visceral injuries when the kidneys themselves are traumatized (1,2).
Each kidney and its adjacent adrenal gland are contained within a thin condensation of connective tissue, the perirenal or Gerota’s fascia. This fascial envelope is circumferentially intact, with the exception of an inferior hiatus that allows for exit of the ureter. Although it does not provide mechanical protection, Gerota’s fascia is an important anatomic barrier against the extension of primary renal tumors—including Wilms’ tumor—to adjacent organs. It also effectively contains and controls the hemorrhage of most blunt trauma. Variable amounts of fat also surround the kidneys in either a perinephric (within the fascia) or paranephric (outside of the fascia) position, buttressing them in the retroperitoneum. Unfortunately, the paucity of fat in most children makes the organ extremely mobile and more susceptible to contrecoup, flexion, and deceleration injuries.
The kidneys of the child, especially at an early age, are larger with respect to overall body size than those of an adult. This results in a relative abdominal projection that, again, makes them more susceptible to injury. Nomograms have been developed that estimate normal renal size with respect to age, although correlations should be made with overall size. As a general rule, the length of a normal kidney equals that of the adjacent two and one half vertebral bodies. The absence of growth on serial examination should prompt an investigation into cause. In addition to size, the axis of the pediatric kidney provides a clue to abnormal development. Normally, each upper pole is distinctly medial to the lower pole, and lines drawn along the longitudinal
axis of the kidneys typically intersect at the 10th thoracic vertebrae. Vertical or laterally displaced axes can be caused by the upper pole hydronephrosis of a duplex collecting system, tumors of the kidney or adrenal glands, or renal ectopia from incomplete migration or rotation during embryogenesis (see below).
axis of the kidneys typically intersect at the 10th thoracic vertebrae. Vertical or laterally displaced axes can be caused by the upper pole hydronephrosis of a duplex collecting system, tumors of the kidney or adrenal glands, or renal ectopia from incomplete migration or rotation during embryogenesis (see below).
Parenchyma and Collecting System
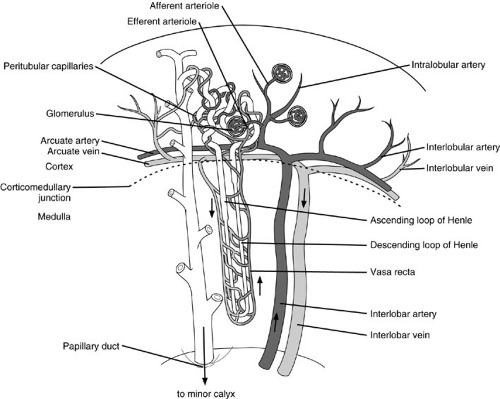
Sagittal sectioning allows an appreciation of the kidney’s macroanatomy (Fig. 95-1). A thick fibrous capsule that adheres to its entire surface maintains the integrity of the renal parenchyma. The capsule is used whenever possible to reinforce the closure of renal wounds because the soft friable parenchyma, like liver, lacks the consistency needed to hold sutures without tearing. Immediately beneath the capsule lies the contiguous outer layer of the kidney, the cortex, which contains the glomeruli, proximal and distal tubules, and collecting ducts. Deep to this lies the central renal medulla, composed of straight portions of the tubules, loops of Henle, the vasa recta, and terminal collecting ducts. The medulla is divided into discrete pyramid-shaped structures that have their base along the corticomedullary junction and are called, appropriately enough, the renal pyramids.
The concavity of the medial surface of each kidney is called the hilum. Here the renal vein, artery, and pelvis are positioned in an anterior-to-posterior direction. The fat-filled space surrounding the renal pelvis and overlying kidney is called the renal sinus. The renal pelvis typically branches into two or three major calyces as it enters the substance of the kidney. Further divisions result in two or three minor calyces. Each renal pyramid (and hence renal lobe) empties into a minor calyx at the papilla, which accounts for the convex impressions seen on pyelography.
Vasculature
There can be significant variation in renal vascularity. Normally, the blood supply to each kidney comes from a single main artery that branches off the aorta, just inferolateral to the superior mesenteric artery. Auxiliary arteries from the aorta and adrenal or gonadal arteries frequently supply the superior and inferior poles, especially to the left kidney. These vessels cross the collecting system and are sometimes implicated in obstructions of the ureteropelvic junction.
Within the renal sinus, the main artery gives off a posterior branch that supplies that segment of the kidney exclusive of the poles. Its anterior limb divides into four branches that feed the apical, upper, middle, and lower segments. Despite any variations that might occur with arterial origins and distribution, there is no collateral blood supply between the individual segments. Each segmental artery is an end artery. Brodel’s line defines a plane between the anterior and posterior branches that, in theory, allows a relatively avascular surgical approach to the center of the kidney.
Unlike its arterial supply, the venous drainage of the kidney freely crosses segmental boundaries. After entering the ascending vasa rectae, venous blood drains into the interlobular veins, and then retraces the arterial path to the main renal vein. Like its arterial counterpart, the renal vein exhibits a great deal of variation. In most cases, the left renal vein, which is the longer of the two, has lumbar, gonadal, and adrenal branches. In contrast, the right renal vein is notoriously short and has no collaterals because the comparable right-sided collateral veins drain directly into the vena cava. This difference in collateral drainage gives the left kidney far more resiliency after ligation or thrombosis of its main vein.
Microanatomy and Functional Correlates
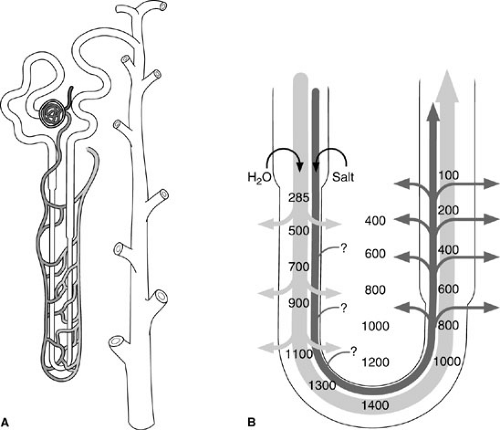
At the microscopic level, the kidney becomes a marvel of the interplay between function and structure. The basic unit of function, the nephron, is composed of a vascular capillary tuft, the true glomerulus, and the glomerular or Bowman’s capsule. In common usage, however, the term glomerulus refers to the combination of capillary tuft and capsule (Fig. 95-2). Each kidney is comprised of about 1 million nephrons whose formation is complete at birth. The kidney is not a resilient organ and the organ’s functional reserve is limited.
Blood flows to the glomerulus by way of the afferent arteriole and exits through the efferent arteriole. Both vessels enter the glomerulus at the vascular pole or hilum directly
opposite the proximal renal tubule at the urinary pole. The glomerulus invaginates the blind-ending renal tubule and the fenestrated epithelial lining of its capillaries, and initiates the ultrafiltration of plasma. Urine ultimately results. The principal driving force regulating glomerular filtration is hydrostatic pressure, a consequence of systemic blood pressure. Glomerular filtration pressure is progressively opposed until ultrafiltration ceases by the hydrostatic pressure in Bowman’s space and colloid osmotic pressure of the arteriole.
opposite the proximal renal tubule at the urinary pole. The glomerulus invaginates the blind-ending renal tubule and the fenestrated epithelial lining of its capillaries, and initiates the ultrafiltration of plasma. Urine ultimately results. The principal driving force regulating glomerular filtration is hydrostatic pressure, a consequence of systemic blood pressure. Glomerular filtration pressure is progressively opposed until ultrafiltration ceases by the hydrostatic pressure in Bowman’s space and colloid osmotic pressure of the arteriole.
The epithelial component of Bowman’s capsule, which surrounds the glomerular tuft and is continuous with the epithelium of the proximal tubule, has both a visceral (lining the capillary tuft) and a parietal (comprising the wall of capsule itself) component. Varying degrees of hematuria, proteinuria, and renal insufficiency result, depending on the severity.
Within the proximal convoluted tubule, the longest portion of the nephron, about 65% of the ultrafiltrate is isotonically reabsorbed. Sodium is actively transported into the peritubular space, and chloride and water passively follow. After passing through the proximal tubule, the ultrafiltrate enters the descending (thin) portion of the loop of Henle accompanied by the adjacent vasa recta. Many loops extend through the medulla to the tips of the papilla with the collecting ducts. Here, a countercurrent multiplier acts to increase the osmotic concentration of the medullary interstitium by actively reabsorbing salt. On its upward swing, ultrafiltrate hypotonicity results because the ascending (thick) loop is impermeable to water, yet salts are actively reabsorbed along their elevated osmotic gradients (Fig. 95-3). The distal tubule and collecting ducts ultimately course back through this same hypertonic medullary interstitium, where water reabsorption is mediated largely under the influence of antidiuretic hormone. Final sodium handling also occurs here, influenced partly by aldosterone.
Henle’s loop ascends to the distal convoluted tubule, which courses alongside the glomerulus of origin. The two are functionally linked by the tubule’s macula densa, a modified group of cells believed to influence the release of renin by the juxtaglomerular apparatus. Multiple collecting tubules drain into individual collecting ducts that run through the medulla and out to the renal papilla, where other collecting ducts are gathered to give the papilla a porous appearance.
EMBRYOLOGY
Congenital anomalies of the urinary tract are common in otherwise normal children, with a rate as high as 3% cited in asymptomatic infants screened with ultrasound (3,4). A significant number of these will require surgical intervention. In addition, because it is synchronously developing with other organs, the urinary tract is often adversely influenced by the same teratogenic, chromosomal, and nonspecific influences that result in syndromes.
The mature kidney evolves through three developmental phases. The pronephros, mesonephros, and metanephros are derived from intermediate mesoderm, which coalesces adjacent to the second through sixth somites. The superior portion of the primordial anlage or nephrogenic cord is composed of primitive tubules known as the pronephros. This appears by the third week of gestation and involutes by week 5.
The medially positioned mesonephros is induced from the mesoderm by the descending mesonephric duct, the wolffian duct, which extends caudally to communicate with the anterior cloaca. The union of duct with mesoderm creates about 40 pairs of mesonephric nephrons, which produce urine between the fifth and tenth weeks of development. Although the mesonephros gradually involutes, vestigial tubules can be found in both genders near the reproductive tracts. The mesonephric duct persists as the vas deferens in males. In females, remnants persist as Gartner’s duct along the anteromedial vaginal wall.
Formation of the mature kidney depends on reciprocal inductive effects of the caudal portion of the nephrogenic cord or metanephros and the ureteral bud. The ureteral bud begins to project from the caudal end of the mesonephric (wolffian) duct during the fourth and fifth weeks of gestation. After the bud unites with the metanephric blastema, its ampulla is induced to repetitively branch for 15 generations. Nephrogenesis is completed by birth, although renal maturation with proximal tubule convolution, collecting tubule elongation, and Henle’s loop extension into the medulla continue afterward. Each nephron follows a sequential order of maturation in segmentation of glomerulus, proximal tubule, and distal tubule.
Nephrogenesis does not occur if the bud is absent or unable to come into close contact with the metanephrogenic blastema. When the tissues are separated enough to prevent epithelial–mesenchymal interaction, differentiation does not occur. After its initial induction by contact, proliferation and morphogenesis of the metanephros continues under the influence of growth factors, extracellular matrix, and proteins.
Abnormalities in the interplay of the ureteral bud and metanephros are implicated in a number of congenital abnormalities of the kidney. For example, ectopic ureters or severely refluxing ureters positioned off the trigone are commonly associated with renal dysplasia or hypoplasia, findings that are rarely associated with a normal ureter. Calyceal diverticula, some cystic variants, megacalycosis, and renal ectopia also undoubtedly have an embryonic basis
where problems with ampullary branching, segmentation of the nephron, or renal ascent can occur. Nevertheless, etiologies are difficult to prove.
where problems with ampullary branching, segmentation of the nephron, or renal ascent can occur. Nevertheless, etiologies are difficult to prove.
ANOMALIES OF NUMBER AND POSITION
Renal Agenesis
Unilateral
Unilateral renal agenesis is a fairly common anomaly (1 per 1,100 in autopsy series and 1 per 1,500 in radiographic reviews). The 2:1 male predominance seen with renal agenesis probably results from the delicate interplay required of the ureteral bud and the wolffian duct. The timing of the error in embryogenesis determines its effects on the urinary tract and associated organ system anomalies (Table 95-1). The most common organ systems involved include cardiovascular (30%), gastrointestinal (25%), and musculoskeletal (14%) (5). As many as one-half of patients with one kidney still have a rudimentary ureter on the affected side, an indication that the problem occurred after the ureteral bud has extended from the wolffian duct, which remains normal. The other one-half of affected males have an absence of the ureter and ipsilateral hemitrigone of the bladder, as well as absence of the wolffian structures (ejaculatory duct, seminal vesicle, and vas deferens) as indicators of an earlier insult in development. In both instances, the ipsilateral gonad is usually normal.
Similar errors in embryogenesis also affect females for apparently the same reasons. Other than giving off the ureteral bud before they involute, the wolffian ducts play a crucial role in the development of the internal genitalia by guiding the müllerian ducts into position along the urorectal septum. It is easy to implicate abnormalities of this sequence with a unicornuate uterus, where the müllerian duct is never properly positioned, or in uterine didelphys, where the normal fusion and canalization of the two ducts is somehow deterred. Not surprisingly, both anomalies are commonly associated with renal agenesis in female patients. For the same reasons, complete absence or hypoplasia of the vagina is also frequently associated with renal agenesis (the so-called Mayer-Rokitansky-Kuster-Hauser syndrome).
Unilateral renal agenesis is occasionally discovered during the evaluation children with multiple organ system anomalies [e.g., syndrome of vertebral defects, imperforate anus, tracheoesophageal fistula, and radial and renal dysplasia (VATER)], serendipitously or in concert with an associated symptomatic genital abnormality such as hydrocolpos. They are most often diagnosed by antenatal ultrasound. After delivery, renal scintigraphy can be used to confirm the diagnosis. Parents are also made aware of the possible genital implications of the finding. Ultrasound can be used to confirm the presence of a cervix and uterus in infants and young girls. Some clinicians have also recommended screening the siblings of affected children, in whom a 10% rate of asymptomatic renal anomalies has been reported.
Until more recently, it was believed that children with solitary kidneys experienced no increased nephrologic risk. Compensatory hypertrophy of the single moiety occurs, and life expectancy is assumed to be normal. However, concerns have been raised about the hyperfiltration load assumed by the remaining kidney.
TABLE 95-1 Common Associations with Renal Agenesis. | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Bilateral
Bilateral renal agenesis is less common than its unilateral counterpart (1 in 4,800 births). Male patients are more commonly affected (75%), although the wolffian derivatives are usually normal. Failure of the ureteral bud is typically implicated because an absent or atretic ureter is found in nearly 90% of cases. An increased incidence of imperforate anus and spina bifida suggests a regional disturbance of the cloaca in some children. In the remainder, a 10% incidence of testicular agenesis in the presence of intact wolffian structures points to a dual insult to the renal and gonadal anlagen in the dorsal coelom.
Anhydramnios or oligohydramnios during pregnancy is usually the harbinger of bilateral renal agenesis. Antenatal ultrasound confirms absence of the kidneys and no evidence of bladder filling after a prolonged examination. Although false-positive results have been reported, consideration is given to termination of pregnancy because of the poor prognosis. Intrauterine compression of the fetus results in the classic stigmata of Potter syndrome (clubbed feet, bowed legs, loose skin, and prominent epicanthal fold of the cheek) and severe pulmonary hypoplasia. Stillbirths result in one-half of the cases, whereas the remaining infants die during the first day of life from pulmonary distress. Anuria and gradual renal failure are predictable for the occasional child who remains alive for a slightly longer period of time. Normal newborns should void during the first 24 hours. Anuria is occasionally prolonged by tocolytic agents given the mother or by spinal flexion from traumatic birth. Renal agenesis is suggested by more prolonged anuria, especially without a distended bladder. Ultrasound is usually diagnostic, although renal scintigraphy can also be performed for confirmation. The risk of similar involvement in subsequent pregnancies has been estimated at 2% to 5%, underscoring the need for an autopsy of any baby believed to have this diagnosis.
RENAL FUSIONS AND ECTOPIA
Early errors in development can adversely affect the ascent and rotation of the metanephros and result in an impressive array of anomalies in position and configuration (6). Despite their distorted configurations, most of these renal anomalies never become clinically significant. However, they should serve as a caution to clinicians because affected children often have abnormalities of other organs.
Horseshoe Kidney
Horseshoe kidneys are the most common fusion anomaly, with an incidence of 1 in 400. A 2:1 male prevalence exists. Girls with Turner’s (45XO) syndrome (60%) and children with trisomy 18 (21%) are also prone to this anomaly. Fusion of the lower poles is found in more than 90% of cases. An isthmus of functioning renal tissue is usually found anterior to the great vessels, just below the inferior mesenteric artery. Renal vasculature is highly variable, and only 30% of horseshoe kidneys have a single renal artery. The remainder receives additional branches from the aorta or the hypogastric, middle sacral, and common iliac arteries.
Most ectopic and fused kidneys are malrotated. The ureters are anteriorly directed and draped over the inferior poles and isthmus of the anomaly. Variable degrees of hydronephrosis are the rule, but full-blown obstruction exists in one-third or less of patients. Stasis does present a problem, however, and calculi ultimately develop in about 20% of patients. When symptoms do occur, vague abdominal pain, a palpable mass, or urinary infections are common presenting signs. A renal scan or intravenous pyelogram (IVP) is diagnostic.
Long-term survival is unaffected by the presence of a fusion or ectopia. However, these kidneys are more prone to trauma and can be difficult to operate on because of their highly variable vasculature. Depending on the pelvic or renal surgery that is planned, arteriography can provide information about the blood supply to the kidney that may be crucial to success. Voiding cystourethrography is also indicated in any symptomatic child with a horseshoe kidney. Vesicoureteral reflux is seen in one-half of these children, and presents a risk factor for infection and upper tract damage in an already abnormal kidney. In addition, for reasons that remain unclear, there is an increased incidence of nephroblastomas and Wilms’ tumor in horseshoe kidneys.
Crossed Renal Ectopia
Crossed renal ectopia is less common than horseshoe kidney (1 in 1,000) and also preferentially affects male patients (2:1). Four categories lend order to what is sometimes bizarre anatomy. In decreasing order of frequency, these include crossed fused (Fig. 95-4A), crossed nonfused (Fig. 95-4B), solitary crossed (Fig. 95-4C), and bilateral crossed (Fig. 95-4D). Crossed fusions constitute 90% of the variants seen, and the left kidney is the ectopic moiety 75% of the time. In most cases, the orthotopic kidney and ureter are normally positioned. Its lower pole, however, is fused to the upper pole of the crossed ectopic kidney, whose ureter is normally inserted in the bladder on the contralateral side. In less well-defined cases, the ureteral origins remain unchanged, but both kidneys meld together on one side.
Other Renal Ectopias
Whenever a kidney occupies an abnormal position, it is classified as ectopic. A compilation of series suggests an overall occurrence of about of 1 in 900. Pelvic ectopia, in
which the kidney is opposite the sacrum, is the most common type (1 per 3,000). Additional ascent yields lumbar (near the sacral promontory) and abdominal (above the iliac crest) variants. Because of its different embryologic origin, the adrenal gland is normally positioned, regardless of the position of the kidney.
which the kidney is opposite the sacrum, is the most common type (1 per 3,000). Additional ascent yields lumbar (near the sacral promontory) and abdominal (above the iliac crest) variants. Because of its different embryologic origin, the adrenal gland is normally positioned, regardless of the position of the kidney.
 FIGURE 95-4. Four common variants of crossed renal ectopia: (A) crossed fused, (B) crossed nonfused, (C) solitary crossed, and (D) bilateral crossed. |
Ectopic kidneys often are dysmorphic and smaller than normal, yet most are asymptomatic despite their abnormal location. Vascularity typically emanates from anomalous branches of the adjacent great vessels. Presentations include hydronephrosis, obstruction, calculi, infection, vague abdominal pain, and posttraumatic hematuria. Moieties positioned over the sacrum may be difficult to visualize. Renal scintigraphy, computed tomography (CT), and ultrasonography are other options in imaging that can be used to make the diagnosis.
Other anomalies (up to 85%) commonly occur with ectopic kidneys (Table 95-2). Because ipsilateral vesicoureteral reflux is a frequent finding (70%), voiding cystourethrography is recommended to complete the workup
of affected children. In addition, the contralateral kidney is abnormal in as many as 50% of patients, causing some investigators to implicate a teratogen in the etiology. Finally, the high incidence of müllerian malformations in girls (45%), including duplications of the vagina and a unicornuate or bicornuate uterus, suggests a problem with the ureteral bud early in development.
of affected children. In addition, the contralateral kidney is abnormal in as many as 50% of patients, causing some investigators to implicate a teratogen in the etiology. Finally, the high incidence of müllerian malformations in girls (45%), including duplications of the vagina and a unicornuate or bicornuate uterus, suggests a problem with the ureteral bud early in development.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree