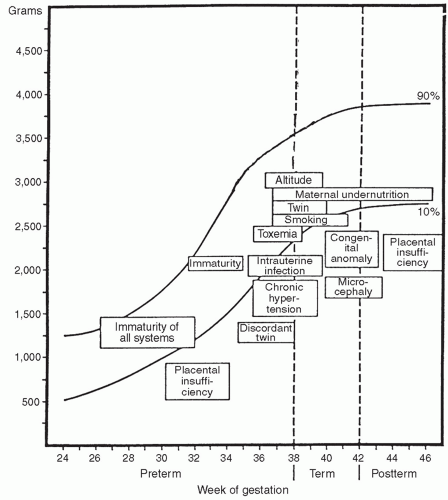
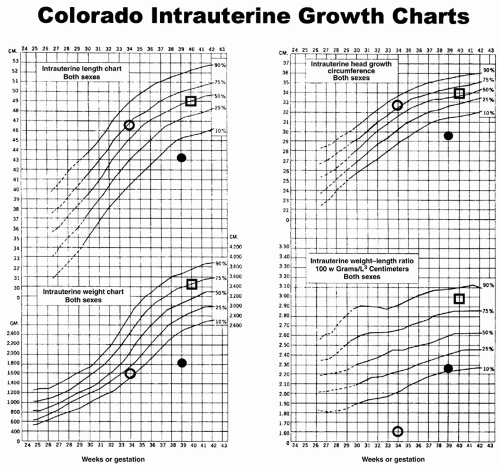
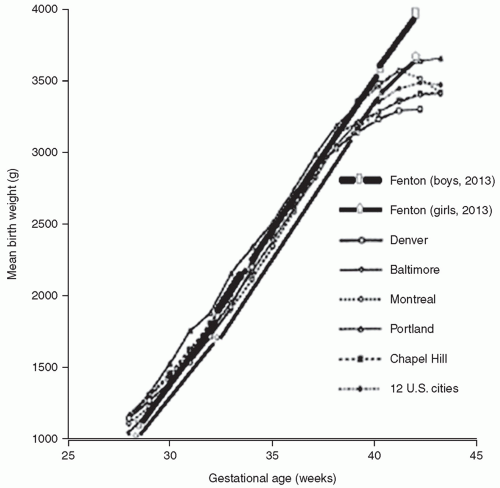
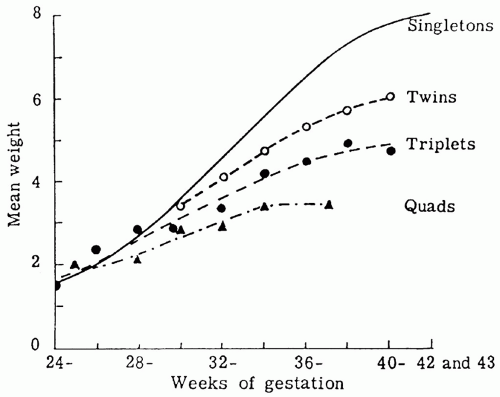
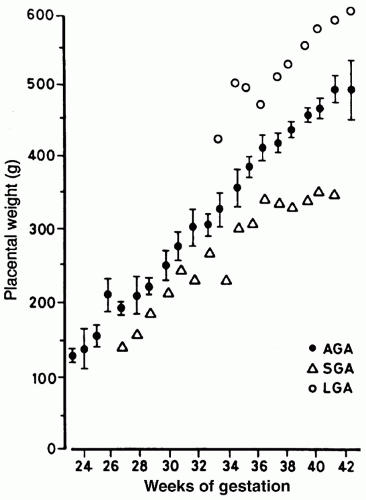
growth of all body organs is restricted, whereas decreased fetal nutrient supply later in gestation primarily restricts growth of adipose tissue and skeletal muscle.
TABLE 23.1 Classification of Fetal Growth | ||||||||
|---|---|---|---|---|---|---|---|---|
|
perfusion, and inadequate nutrient substrate uptake. Preeclamptic women have poor endometrial vascular support for growth of the placenta, leading to placental growth failure, fetal nutrient deficit, and IUGR (13). Fetal hypoglycemia, hypoxemia, and acidosis usually are present in such cases of poor placental development and perfusion. These factors lead to increased production of prostaglandins and the activation of labor-promoting cytokines, leading to preterm delivery (14). Women at the age limits of childbearing produce IUGR infants who often are born prematurely. Nutritional, uterine, and vascular mechanisms may be common in these situations. Young, still-growing adolescent girls appear less capable of mobilizing fat reserves in late pregnancy, apparently reserving them instead for their own continued development (15). IUGR in cases of maternal smoking and substance abuse may result from reduced placental blood flow, inhibition of uteroplacental vascular development, or direct fetal toxicity.
abnormalities have been shown to develop in a sequential fashion as placental insufficiency progressively worsens, and may predict risk of acidosis and perinatal mortality as well as help to predict optimal timing of delivery (16).
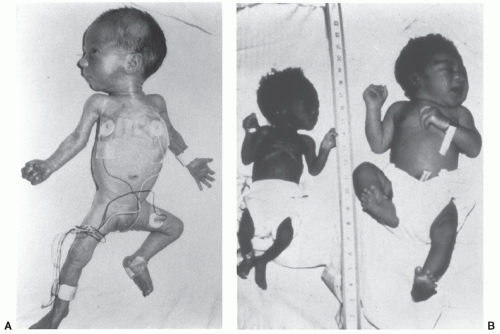 FIGURE 23.3 Preterm, SGA infant at 34 weeks of gestation (left), severely SGA infant at 39 weeks (middle), and AGA infant at 40 weeks (right). |
TABLE 23.2 Maternal Conditions Associated with Intrauterine Growth Restriction and Preterm Delivery | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 23.3 Factors Determining Variance in Birth Weight | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
impact on fetal growth and the severity of IUGR. This is because changes in maternal nutrition, unless extreme and prolonged, do not markedly alter maternal plasma concentrations of nutrient substrates or the rate of uterine blood flow, the principal determinants of nutrient substrate delivery and transport to the fetus by the placenta. Human epidemiologic data from conditions of prolonged starvation, and nutritional deprivation in experimental animals, indicate that even severe limitations in maternal nutrition limit fetal growth only by 10% to 20%. Epidemiologic data from the Dutch during the Hunger Winter of 1944 showed an average reduction in fetal weight at term of 300 g (31). In animal models, experimental restriction of calorie and protein intakes to less than 50% of normal for a considerable portion of gestation are needed before marked reductions in fetal growth are observed. Such severe conditions often result in fetal loss before the impact of fetal growth rate in late gestation and fetal size at birth are manifested. Attempts to increase fetal weight gain with maternal nutritional supplements have produced mixed results. Higher caloric feeding increases fetal adiposity, not growth of muscle mass or gain in length or head circumference (32). In contrast, high protein supplements tend to produce delayed fetal growth (32). Mechanisms responsible for these disparate outcomes are not known, though insights resulting from experimental amino acid infusions into pregnant sheep have been proposed, including competitive inhibition among coinfused amino acids for common transporters across the placenta, as well as a possible mismatch between amino acid supply and fetal growth factor availability (i.e., insulin and IGF-1, which also are reduced in IUGR fetuses), limiting anabolic capacity even when amino acid supply might be increased (33).
TABLE 23.4 Drugs Associated with Intrauterine Growth Restriction | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 23.5 Placental Growth Disorders that Lead to or Are Associated with Intrauterine Growth Restriction | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
increases cytotrophoblast proliferation over differentiation and invasion, thus setting the stage for deficient placental development that can result in deficient nutrient and growth factor supply to the fetus, producing fetal growth restriction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree