Inflammation, Perinatal Morbidity, and Long-Term Outcome
Olaf Dammann
T. Michael O’Shea
▪ INTRODUCTION
Inflammation refers to an array of molecular processes that plays a role in the pathophysiology of certain pregnancy and neonatal disorders. Perinatal inflammation could link specific maternal and pregnancy disorders to morbidity in the offspring, including acute organ dysfunctions, developmental impairments and, possibly, programming for adult diseases. Therefore, it is a potential target for prevention efforts to improve pregnancy outcome. In addition, inflammation markers have potential as early predictors of neonatal disorders, such as sepsis and necrotizing enterocolitis (NEC), and as biomarkers to monitor disease progress and the efficacy of medical interventions.
Information about the possible effects of perinatal inflammation on the neonate derives from studies in which perinatal inflammation is quantified using data about clinical initiators of inflammation, histologic examination of the placenta and umbilical cord for evidence of immune cell infiltration, and measurements of inflammation-related proteins in amniotic fluid, maternal blood, fetal blood, and neonatal blood. Archived samples have been used for biomarker epidemiologic studies of brain-related outcomes that can be diagnosed only years after delivery (1,2,3).
We begin this chapter by reviewing fundamental biologic concepts of inflammation, including the emerging concept of intermittent or sustained systemic inflammation (ISSI). Then, we will discuss exposures that could contribute to perinatal inflammation and thus to acute and persistent dysfunctions and describe adverse neonatal outcomes related to inflammation. We conclude by describing interventions that might modify inflammatory processes in the perinatal period, thereby improving health and developmental outcomes.
▪ INFLAMMATION
Three aspects of inflammation are particularly important to keep in mind in the neonatal scenario: innate immune phenomena are a double-edged sword, the relationship between innate and adaptive immune mechanisms, and the evolving concept that prolonged exposure to ISSI might be what places the newborn at highest risk.
Innate Immune Response
The innate response consists mainly of an early response characterized by the generation of acute-phase proteins (4). Immune cells have pattern recognition receptors (PRRs). Perhaps, the most widely known PRRs are the Toll-like receptors (TLRs) that are expressed on the cell surface and within endosomes. These receptors are activated by pathogen-associated molecular patterns (PAMPs), for example, by lipopolysaccharide (LPS), a marker expressed on the cell surface of gram-negative bacteria. TLR stimulation initiates cell activation, gene expression, and acutephase protein (5). TLR activation plays an important role in neonatal sepsis and other morbidities (6). Perhaps, the best studied acute-phase signals associated with the innate immune response in newborns are C-reactive protein (CRP) and proinflammatory cytokines and chemokines. These proteins peak within 1 to 2 days of a strong proinflammatory stimulus such as neonatal surgery, and different patterns (including nonresponse) are observed with different indications for surgery (7). Procalcitonin, CRP, serum amyloid alpha (SAA), and other proteins are candidate biomarkers for neonatal sepsis and NEC (8). Although it is generally assumed that term newborns have a more vigorous innate response than do preterm newborns, multiple studies point to the contrary. Even among extremely preterm infants, the most prominent pattern of developmental regulation is a decrease of inflammation-related protein concentration with increasing gestational age, regardless of placenta inflammation (9). This, together with the recognition that inflammation plays a role in neonatal disease pathogenesis, helps explain the decreasing prevalence of neonatal morbidity with increasing gestational age.
Innate and Adaptive Immunity
It is likely that the innate immune response activates and perhaps even regulates the adaptive immune system (10). Therefore, it is likely that the current research focus on the innate immune response in newborns will soon be replaced by a focus on the relationships between the innate and adaptive immune systems. We have growing evidence that, for example, neonatal brain white matter damage can result from proinflammatory feedback loops between both systems (11). Such mechanisms could be a likely explanation for the seminal observation that proin-flammatory cytokines are elevated in school-age children with cerebral palsy compared to controls (12). This observation leads to the question of whether perinatal systemic inflammation persists years after initiation. Several potential mechanisms for persisting inflammation have been proposed (13) (Fig. 42.1).
Intermittent or Sustained Systemic Inflammation
A rapidly mounted systemic inflammatory response can be an effective defense against microbial invasion and should follow a set pattern beginning with an initiation (proinflammatory) phase, rapidly followed by an adaptive (anti-inflammatory) phase, and finally a resolution (restoration of homeostasis) phase (14). Failure of inflammation resolution processes, however, leads to dysregulated and prolonged inflammation, which can damage organs and contribute to the development of a whole host of chronic diseases in adults (15). Data from observational and experimental studies document that, once initiated, the fetal/neonatal inflammatory response can be present for long intervals (16), but we do not yet know if this prolonged systemic inflammation is intermittent or sustained. The term “intermittent or sustained systemic inflammation” has been suggested for this neonatal risk factor (17), implying that resolution is possible. Others do not hesitate to eliminate this possibility when using the word “persistent” in describing among surgical patients what they call PICS, persistent inflammation, immunosuppression, and catabolism syndrome (18).
Following an intravenous inflammatory stimulus (i.e., LPS), healthy young adult volunteers have tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 concentrations that peak at about 2 to 2½ hours and return to baseline within 12 hours (19). In preterm newborns, however, some indicators of systemic inflammation (proin-flammatory cytokines) are elevated after birth much longer than would be expected based on their half-life in adults (20,21). We still do not know the half-life of inflammation-related proteins in preterm newborns. Consequently, we do not yet know if ISSI reflects nothing more than a developmentally regulated very prolonged catabolic process. Whatever the pathogenetic mechanism associated with ISSI, elevated levels of inflammation-related proteins in blood collected on postnatal days 7 and 14, especially when sustained, are associated with impaired mental and motor development at age 2 years (22) (Fig. 42.2).
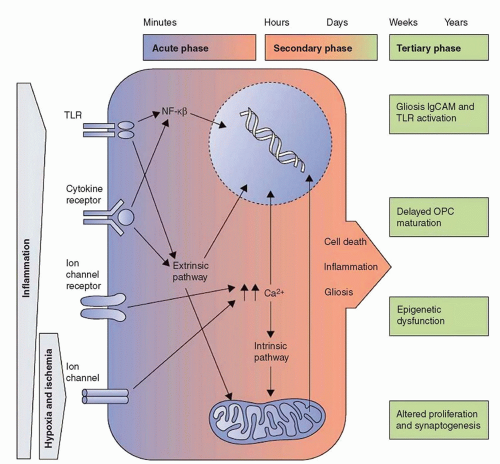 FIGURE 42.1 Outline of the acute, secondary, and tertiary damage phases in cerebral palsy. From Fleiss B, Gressens P. Tertiary mechanisms of brain injury: a new hope for treatment of cerebral palsy? Lancet Neurol 2012;11(6):556-566. |
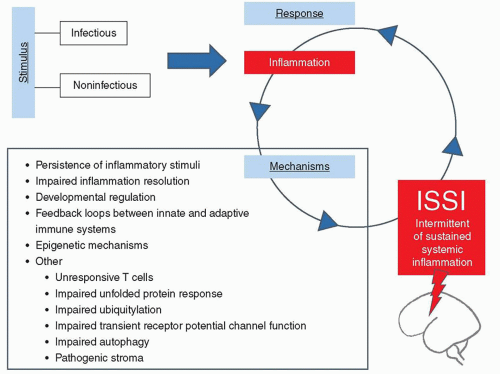 FIGURE 42.2 Proposed list of candidate mechanisms linking infectious and noninfectious stimuli, the initial inflammatory response, and subsequent intermittent or sustained inflammation (ISSI). From Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res 2014;75(3):376-380. |
▪ PROINFLAMMATORY EXPOSURES
See Table 42.1.
Inflammatory Exposures Prior to Labor
Even before the onset of labor, certain maternal conditions appear to contribute to neonatal systemic inflammation. Extremely preterm infants whose mothers have vaginitis are more likely than peers whose mothers do not to have vaginitis to exhibit elevations of inflammation-related proteins, similar to those associated with intrauterine inflammation (23). Prepregnancy maternal obesity also is associated with elevations of inflammation-related proteins in neonatal blood but only among neonates delivered after preeclampsia or a fetal indication, such as growth restriction (37). The association of intrauterine growth restriction and inflammation has been described in term (27), as well as preterm (38), newborns.
Inflammation and Preterm Labor
Microorganisms are recovered frequently from placentas from pregnancies delivered prematurely (24). The prevalence of colonization of the placenta is highest in association with preterm labor (53%) and lowest with preeclampsia leading to cesarean section (25%). Among pregnancies complicated by preterm labor, the colonization rate varies from about 80% at 23 weeks of gestation to about 40% at 27 weeks of gestation. The recovery from the placenta of Actinomyces, Prevotella bivia, Corynebacterium sp., Escherichia coli, Peptostreptococcus magnus, multiple species of streptococci, and Mycoplasma sp., including Ureaplasma urealyticum, is associated with highgrade chorionic plate inflammation and fetal vasculitis (39).
Researchers have grouped pregnancy disorders that precede extremely preterm delivery into disorders that are associated with intrauterine inflammation, indicated by histologic chorioamnionitis, and disorders that are associated with histologic evidence of dysfunctional placentation, such as infarcts, intervillous fibrin, fetal stem vessel thrombosis, and decidual hemorrhage and fibrin deposition (40,41). In a large cohort of births at less than 28 weeks’ gestation in the United States, 79% were categorized as associated with intrauterine inflammation and 21% as associated with preeclampsia/fetal indication. Disorders associated with intrauterine inflammation include preterm labor, prelabor premature rupture of the membranes, placental abruption, and cervical incompetence. These conditions are associated with the recovery of microbes from the placental parenchyma, histologic evidence of inflammation in the placenta and umbilical cord (funisitis), and inflammation markers, such as cytokines, in maternal, fetal, and neonatal blood (40,41).
Disorders associated with preterm delivery but not with intrauterine infection include preeclampsia and fetal indications for delivery, the most prominent of which is intrauterine growth restriction. These disorders are not associated with either inflammation of the placenta or recovery of microbes from the placenta. Placentas from pregnancies complicated by severe preeclampsia have lower concentrations of acute inflammatory proteins, such as IL-1β, IL-18, IL-6, and TNF-α, as compared to placentas from pregnancies complicated by preterm labor or prelabor premature rupture of membranes (25). Whether the infant is born by vaginal or caesarean delivery probably does not influence placental concentrations of inflammation-related proteins (25).
TABLE 42.1 Proinflammatory Exposures for Fetuses and Neonates | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||
Preterm delivery attributed to intrauterine inflammation is associated with a fetal inflammatory response, as indicated by elevated blood IL-6, and a fetal inflammatory response is predictive of imminent onset of preterm labor (42). Preterm birth is associated with increased levels of proinflammatory cytokines in fetal and cord blood (31). Intrauterine inflammation is associated with the offspring’s having elevated blood levels, on the first postnatal day, of inflammation-related proteins, including cytokines, cytokine receptors, acute-phase reactants, chemokines, adhesion molecules, and metalloproteinases.
Elevation of inflammation-related proteins is not found in blood collected on the first postnatal day from extremely preterm infants delivered because of preeclampsia or fetal indication/growth restriction (23). However, by the 14th postnatal day, infants with fetal growth restriction have elevated levels of CRP, IL-1β, IL-6, TNF-α, IL-8, monocyte chemotactic protein-4 (MCP-4), intercellular adhesion molecule-1 (ICAM-1), intercellular adhesion molecule-3 (ICAM-3), E-selectin (E-SEL), matrix metalloproteinase-9 (MMP-9), vascular endothelial growth factor receptor-2 (VEGF-R2), and/or insulin-like growth factor-binding protein-1 (IGFBP-1) (38).
In the term and near-term neonate, complications related to placental insufficiency can initiate inflammation. The fetus swallows up to 450 mL of amniotic fluid each day, and microbes ingested from the amniotic fluid are present in meconium. Thus, meconium aspiration can expose the fetal and newborn lung to microbes, initiating a local inflammatory process in the lung. Organ damage to the brain, kidney, and intestine resulting from intrapartum asphyxia can initiate inflammation.
Postnatal Inflammatory Exposures
Postnatal exposures are particularly important initiators of inflammation among extremely preterm infants. Among the most prevalent is mechanical ventilation. Blood concentrations of inflammation-related proteins, particularly IL-8, MCP-1, and ICAM-1, increase with increasing duration of mechanical ventilation (32). In contrast, the blood concentration of RANTES (regulated upon activation, normal T-cell expressed, and [presumably] secreted), which is associated with a decreased risk of bronchopulmonary dysplasia (33), decreases with increasing duration of mechanical ventilation (32). The same is true for vascular endothelial growth factor, an inflammation-related protein that probably is involved in the reparative phase of lung inflammation.
Within 7 postnatal days, infants who eventually are diagnosed with bronchopulmonary pulmonary dysplasia already have higher blood levels of multiple inflammation-related proteins, most prominently, ICAM-1, TNF-α, IL-1β, and MCP-1 (33). The presence of inflammation-related proteins in blood could be due in part to intrapulmonary synthesis, as tracheal secretions from infants treated with mechanical ventilation contain inflammation-related proteins, and the levels of these proteins are increased among infants who subsequently develop bronchopulmonary dysplasia (26).
In many infants, colonization or infection of the lung probably contributes to the systemic inflammation that has been associated with mechanical ventilation. In particular, Ureaplasma infection in utero appears to initiate a fetal systemic inflammatory response that increases the risk of neonatal chronic lung disease and brain disorders (34). Postnatal respiratory tract colonization with Ureaplasma is associated with increased concentrations of IL-1β in tracheal aspirates, which, in turn, is associated with an increased risk for chronic lung disease (35). Aggressive mechanical ventilation, even in the absence of microbial infection, is associated with inflammatory changes in the lung (28,36).
Culture-confirmed bacteremia is associated with systemic inflammation, but culture-negative (suspected) sepsis is not. In the large multicenter cohort recruited for the ELGAN Study, culture-confirmed early sepsis (in the first postnatal week) was associated with elevated blood CRP and IL-8 on the first postnatal day, and elevations of SAA, TNF-α, ICAM-1, and VEGF-R2 around the 7th postnatal day, but was not associated with elevation of inflammation-related proteins around the 14th postnatal day. In contrast, infants with late bacteremia (after the first postnatal week) did not have evidence of an inflammatory response on the first postnatal day, but in the second week of life had elevated levels of several inflammation markers, including CRP, SAA, IL-8, IL-6, TNF-α, ICAM-1, E-SEL, VEGF-R2, macrophage inhibitory protein-1β (MIP-1β), interferon-inducible T-cell alpha-chemoattractant (I-TAC), and tumor necrosis factor receptor-2 (TNF-R2). The inflammatory response was stronger for late as compared to early bacteremia (29). Elevations of CRP, SAA, and IL-8 in blood, occurring around postnatal days 7 and 14, have also been found among infants with NEC, and elevations of CRP and SAA have been found among infants with intestinal perforation (30).
Extremely preterm infants with intraventricular hemorrhage have a more intensive systemic inflammation response when the hemorrhage is accompanied by white matter damage. Intraventricular hemorrhage is associated with elevations of IL-8, MCP-1, vascular cell adhesion molecule-1 (VCAM-1), MMP-1, and MMP-9, in the first 2 postnatal weeks. These same proteins, except for VCAM-1 and MMP-1, are elevated also among neonates with intraventricular hemorrhage accompanied by white matter damage; in addition, these neonates have elevations of CRP, SAA, TNF-α, ITAC, ICAM-1 and ICAM-3, and MIP (43). The systemic inflammatory response that is associated with white matter damage could result from local production of inflammation-related proteins in the damaged brain, with subsequent movement from the brain into blood. Alternatively, a large body of evidence suggests that systemic inflammation might increase the risk of white matter damage (44,45,46).
▪ PERINATAL INFLAMMATION AND ACUTE NEONATAL OUTCOMES
Chorioamnionitis, which often is associated with a fetal systemic inflammatory response, is associated with multiple acute physiologic changes during the neonatal period. Among very-low-birth-weight infants, histologic chorioamnionitis is associated with greater illness severity (61), as reflected in standard measures such as the Score for Neonatal Acute Physiology (47). Conflicting evidence exists on the relationship of placental markers of inflammation, funisitis, and chorioamnionitis, to blood pressure (48,49). Placental inflammation is associated with higher concentrations of blood IL-6 and higher proportions of immature neutrophils in umbilical cord, and these inflammation markers are inversely related to systolic and diastolic blood pressure (51). However, in a large cohort of extremely preterm infants, funisitis and chorioamnionitis were not associated with hypotension requiring treatment (49). Extremely preterm infants with high blood PaCO2 (highest quartile) and those with low blood pH (lowest quartile) are more likely to have intermittent or sustained elevations of inflammation markers in their blood during the first 2 postnatal weeks (50). Infants with elevations of CRP have lower peripheral tissue oxygenation, based on near-infrared spectroscopy (62).
Perinatal inflammation is associated with altered function of multiple organs. Increased numbers of inflammatory cells and TLR-2 have been described in the skin of fetuses exposed to microbial invasion of the amniotic cavity (52). Reduced thymic size (53) and microscopic findings of reduced thymocytes number and degenerative processes of Hassall bodies (63) are found more frequently among infants born to mothers with chorioamnionitis than gestational age-matched peers. Both fetal inflammation and thymic involution are associated with an increased risk of ultrasound-defined cerebral white matter damage (54




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


