Germ Cell Tumors
Michael A. Skinner
Division of Pediatric Surgery, Duke University Medical Center, Durham, North Carolina 27710.
One of the great mysteries of human embryology is how the single-cell pluripotential zygote, formed by the union of two germ cells, is able to differentiate into the multiplicity of different tissues seen in the fully developed human. This genetic versatility is in contrast to the equally fascinating notion that in the fully developed organism, although each cell in the body contains every gene, relatively few of these genes are actually expressed within a particular tissue type. Clearly, at some point during embryonic development, a regulatory process is initiated to ensure the expression of only those genes actually necessary for the formation and function of a particular organ.
This exquisite genetic regulation is circumvented in tumors arising from primitive germ cells. Thus, germ cell neoplasms appear to recapitulate some aspects of embryonic development so they can be composed of a mixture of tissue types representative of many different organs in various stages of maturity. This fascinating aspect of germ cell neoplasms distinguishes them from other pediatric tumors.
EPIDEMIOLOGY
Germ cell tumors account for about 3% of neoplasms in children and adolescents. Because of the bias in pediatric centers toward the treatment of malignant tumors, it has been difficult to determine the overall incidence of these neoplasms; the number of benign lesions has typically been underrepresented in large published series. Based on recent population-based studies, germ cell tumors in all locations have been estimated to occur at an incidence of about four to six cases per 1 million children (1). The report from the Children’s Tumor Registry in Manchester, England, noted that the incidence of these tumors has significantly increased since the mid-1970s.
About 25% to 33% of germ cell neoplasms are malignant, and the ratio of female patients to male patients is roughly 3:1 in children younger than age 15. In large population-based studies, the mean age of the patients at the time of diagnosis varies according to the anatomic location. Ovarian tumors are diagnosed in girls at a mean age of about 10 to 15 years, whereas testicular lesions in children occur from infancy into the teenage years. Most sacrococcygeal tumors are seen in early infancy, but they rarely can develop as late as adolescence. Most mediastinal and retroperitoneal germ cell tumors are diagnosed after age 2.
Risk factors for the development of gonadal germ cell neoplasms include the presence of dysgenetic gonads, a family history of the disease, and cryptorchid testes (1). Most germ cell neoplasms, however, occur in the absence of these findings. Klinefelter’ syndrome has been strongly associated with the presence of extragonadal tumors, especially of the mediastinum. There has been speculation about the possibility that germ cell tumors may be a rare manifestation of the Li-Fraumeni syndrome. Although no firm data link any maternal factors with the subsequent formation of germ cell tumors, the presence of an infection during pregnancy has been implicated. Also, there appears to be a higher incidence of these tumors among twins.
EMBRYOLOGY, PATHOLOGY, AND ANATOMY
Germ cell neoplasms are believed to originate from the primordial germ cells. Embryologically, these cells become visible during the fourth week of gestation as they form in the yolk sac near the origin of the allantois. As the craniocaudal fold of the embryo forms, the dorsal portion of the yolk sac is internalized. During this process, the primitive germ cells migrate through the dorsal mesentery of the hindgut to rest in the gonadal ridges, where they ultimately reside in the developing gonad. For unknown reasons, these cells occasionally miss their proper destination and, if transformed, can multiply in an extragonadal location. These locations are usually somewhere in the midline, ranging from the sacrococcygeum to an intracranial site.
The histologic classification of pediatric germ cell tumors follows:
Germinoma
Teratoma
Mature
Immature
Embryonal carcinoma
Yolk sac tumor (endodermal sinus tumor)
Choriocarcinoma
Gonadoblastoma
Mixed germ cell tumor
An understanding of the various differentiation pathways available to the pediatric germ cell is helpful in comprehending the nomenclature of these pathologic subtypes. Recall that the embryo is not the only product of gestation. Rather, three separate types of gestational tissue are derived from the embryonic zygote, which is itself formed by the fusion of the male and female germ cells. These three tissue types are the yolk sac, the placenta and its chorionic membranes, and the fetus. A germ cell neoplasm can recapitulate any of these organ systems.
For example, as illustrated in Fig. 40-1, a primordial germ cell can become transformed before undergoing any further differentiation, resulting in a pure germ cell tumor. Such a lesion is called a seminoma if in the testes, dysgerminoma in the ovary, or germinoma if arising in an extragonadal site. Alternatively, the primordial germ cell can exhibit some differentiation into one of the three gestational elements before transformation. In cases in which differentiation into somatic elements occurs, tissue from one or more of the embryonic somatic cell layers exists and a teratoma is seen. Cells that differentiate along extraembryonic lines can recapitulate tissue reminiscent of trophoblast, leading to the formation of a choriocarcinoma. Finally, a yolk sac tumor, also called an endodermal sinus tumor, can result from the malignant transformation of a germ cell into the yolk sac gestational elements. The yolk sac tumor is the most common form of malignant germ cell neoplasm found in children.
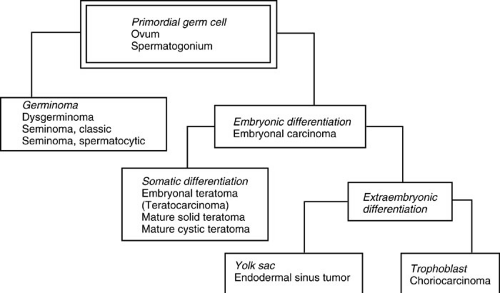 FIGURE 40-1. Diagram showing the derivation of different germ cell tumor subtypes. (Adapted from Dehner LP, ed. Pediatric pathology, 2nd ed. Baltimore: Williams & Wilkins, 1987:764.) |
Historically, pathologists have demanded that tissues from each of the three somatic cell layers be recognized before a tumor is classified as a teratoma. This requirement has been relaxed, and most pathologists now require the presence of only two embryonic layers in these tumors. In rare cases, teratomas contain elements from only one somatic layer. Histologically, teratomas are further classified according their stage of immaturity, which is the degree to which they recapitulate undifferentiated fetal tissues as opposed to mature somatic tissue. A commonly used grading system is shown in Table 40-1. In general, the more immature the cells are, the more aggressively the tumor behaves. Overall, most teratomas below grade 3 act benignly, but it is impossible to predict precisely how individual tumors will behave. In very young patients, such as newborns, the presence of immature fetal tissue is to be expected and is of
little prognostic significance. The malignant potential and clinical behavior of a teratoma is related less to the histologic maturity than to the presence of adjacent frankly malignant germ cell elements within the tumor; such tissue is from one of the other malignant subtypes listed earlier.
little prognostic significance. The malignant potential and clinical behavior of a teratoma is related less to the histologic maturity than to the presence of adjacent frankly malignant germ cell elements within the tumor; such tissue is from one of the other malignant subtypes listed earlier.
TABLE 40-1 Histologic Grading of Teratomas. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
The pathologic subtypes and sites of origin in 170 germ cell tumors from the Federal Republic of Germany Tumor Registry are shown in Table 40-2 (3). As shown in this population-based series, most germ cell tumors arise in the testicle or ovary. Moreover, teratomas are the most common histologic subtype among extragonadal germ cell tumors; these lesions usually arise in the sacral region, the mediastinum, peritoneum, and intracranially. Rarely, germ cell neoplasms appear in the stomach, the liver, or within the urinary tract. About 25% of teratomas are malignant; a histologic example is shown in Fig. 40-2. Although any form of germ cell malignancy can be seen in association with a teratoma, the yolk sac or endodermal sinus tumor is present most frequently.
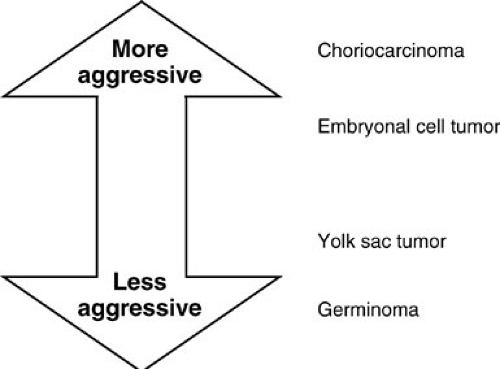
The unequivocally malignant germ cell tumors, as shown in Fig. 40-3, range from the usually well-behaved germinomas to the very aggressive choriocarcinomas. In about 11% of malignant germ cell tumors, excluding teratomas, more than one pathologic subtype is seen. Germinomas occur most frequently within gonadal sites or in the pineal region of the central nervous system. Pure yolk sac tumors are found most frequently in the ovaries, testes, or sacral region. This pathologic subtype is also commonly seen with sacrococcygeal teratomas. Embryonal carcinomas are rare and usually arise in the testes. Gonadoblastoma, which acts like a benign lesion, occurs almost exclusively in dysgenetic gonads in association with the XY genotype (1). In about 25% of these cases, there is an associated focus of malignant germinoma.
TABLE 40-2 Site and Histologic Distribution of 170 Pediatric Germ Cell Tumors from the Federal Republic of Germany Pediatric Tumor Registry, 1966–1984. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
GENETIC ASPECTS AND SERUM MARKERS
As with any neoplasm, the primary initiating factor in the formation of germ cell tumors is a genetic one; one or a number of genes are abnormally expressed to cause unchecked growth of the tumor. Alternatively, there is the absence of some gene expression that would normally serve to control cellular replication. In many tumors, alterations in the chromosomes have provided valuable clues for determining which genes may be important in the genesis or progression of the neoplasm. For example, tumor suppressor genes have been located in regions of the genome that are absent in the tumor when compared with normal tissue. Usually, a region of one chromosome is missing, leading to the loss of heterozygosity, as demonstrated by informative genetic markers. In other cases, there is the amplification, or an increase in the copy number, of a particular gene in the tumor. This is the case in some neuroblastomas, in which the amplification of the N-myc oncogene is a marker of more aggressive tumor biology.
Investigators have examined the chromosomes of germ cell tumors in the hope of finding clues to the molecular pathogenesis of this disease. In cultured cell lines,
nonrandom abnormalities of chromosomes 1 and 12 have been observed. Further, in studies of resected gonadal and extragonadal germ cell tumors, gains of genetic material in chromosomes 1, 7, 12, 21, 22, or 6 were seen in more than of 70% of cases (4). Other investigators have found consistent genetic rearrangements of chromosomes 1, 12, and 17 in seminomas. Further, loss of heterozygosity has been reported on chromosome 12. In assays of known oncogenes, no consistent abnormalities have been seen in germ cell tumors.
nonrandom abnormalities of chromosomes 1 and 12 have been observed. Further, in studies of resected gonadal and extragonadal germ cell tumors, gains of genetic material in chromosomes 1, 7, 12, 21, 22, or 6 were seen in more than of 70% of cases (4). Other investigators have found consistent genetic rearrangements of chromosomes 1, 12, and 17 in seminomas. Further, loss of heterozygosity has been reported on chromosome 12. In assays of known oncogenes, no consistent abnormalities have been seen in germ cell tumors.
The most consistent cytogenetic abnormality in germ cell neoplasms is amplification in a region of the short arm of chromosome 12. This finding has been termed an isochromosome, designated i(12p), and was seen in more than 80% of tumors in some series (5). Isochromosomes have been noted in tumors of all cell types, and it has been suggested that increased i(12p) copy number may be associated with more aggressive tumors. Further, the abnormality appears to be a relatively specific cytogenetic marker for germ cell tumors because it is seen infrequently in other neoplasms. The high frequency of i(12p) in these tumors provides strong evidence that a gene important in the differentiation or growth of germ cells resides on chromosome 12. It remains unknown how the abnormalities in this segment of the genome influence the initiation or progression of germ cell tumors.
Some germ cell tumors produce and secrete protein products that can be measured in the serum. For example, yolk sac tumors often produce α-fetoprotein (AFP) because the yolk sac is the source of this factor during early embryogenesis. The serum level of this product can also be elevated in patients who have embryonal carcinoma. After complete resection of an AFP-producing tumor, the serum level of the protein should drop at a rate reflecting its normal half-life, which is about 4 days (6). The AFP level can then be measured serially to monitor for recurrence of the tumor. In newborns, the use of AFP in this manner is somewhat complicated because the AFP is normally high in neonates. Reference values for AFP at various ages are shown in Table 40-3.
Another important serum factor produced by germ cell neoplasms is β-human chorionic gonadotropin (β-hCG). Normally produced by the placenta after the successful implantation of the fertilized egg, β-hCG is a marker for germ cell neoplasms that have trophoblastic elements. Choriocarcinomas invariably produce this protein, and the marker is useful both in making the initial diagnosis and in follow-up after surgical treatment. Another serum marker
that is often elevated in germinomas is lactate dehydrogenase, but this finding is relatively nonspecific for germ cell tumors.
that is often elevated in germinomas is lactate dehydrogenase, but this finding is relatively nonspecific for germ cell tumors.
TABLE 40-3 Normal Infant Serum α-Fetoprotein (AFP) Levels at Various Ages. | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
CLINICAL ASPECTS OF GERM CELL TUMORS
Germ cell tumors arise from pluripotential germ cells located throughout the body. Anatomically, the tumors can occur either within the gonads or extragonadally. In clinical series in which pediatric germ cell tumors of all locations and pathologic subtypes were tabulated, about 45% to 50% of the lesions were gonadal in location; the remainder occurred within the soft tissues in various other parts of the body (Table 40-2) (1,3,7).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree