Gastrointestinal Bleeding
Michael G. Caty
B. Robert Gibson
Division of Pediatric Surgery, State University of New York at Buffalo, Women and Children’s Hospital of Buffalo, Buffalo, New York 14222.
Department of Surgery, State University of New York at Buffalo, Erie County Medical Center, Buffalo, New York 14215.
Gastrointestinal (GI) hemorrhage is an alarming situation for both parent and surgeon. Fortunately, GI bleeding in most children is due to benign causes and is usually self-limited (Table 71-1). Nevertheless, an aggressive diagnostic approach to the child with significant bleeding is warranted. A diagnostic approach based on the frequency of age-related causes of bleeding usually results in the establishment of a cause for the bleeding.
INITIAL APPROACH AND RESUSCITATION
The initial steps in the evaluation of the child with GI bleeding are to identify the source as upper or lower, assess the magnitude of the bleeding, and initiate resuscitation of the child.
Upper GI hemorrhage is defined as bleeding that originates proximal to the ligament of Treitz. This is manifested by either hematemesis, melena, or occult blood loss. Lower GI hemorrhage may present with hematochezia, melena, or occult blood loss. Elements of the patient’s history that suggest an upper GI source include preexisting liver disease, recent surgical stress or injury, or a family history of ulcer disease. A recent diarrheal illness, history of weight loss with abdominal pain, or family history of polyps or colon resections may suggest a lower source of GI bleeding. Following examination of the nasopharynx to exclude a non-GI source of bleeding, the initial diagnostic maneuver is to place a nasogastric tube. The absence of blood in the presence of aspirated bile rules out an upper GI source with reasonable certainty. If blood is detected, the nasogastric tube allows lavage of the stomach to assess the rate of bleeding. It also removes blood that would impair endoscopic evaluation.
To direct the diagnostic evaluation and guide the resuscitation, an estimate of the rate of bleeding must be made. An initial impression can be formed from observation of the volume of blood from the nasogastric tube or amount of melenic stool. The most important information comes from the physiologic status of the infant or child. Children tolerate blood loss of less than 10% of blood volume (8 mL per kg) extremely well and may demonstrate only minimal elevation of the pulse rate. Increasing heart rate and the presence of orthostatic hypotension suggest a 10% to 20% loss. The findings of hypotension and poor capillary refill are associated with blood loss in excess of 30% of blood volume. Patients with greater than 10% blood loss should be monitored in an intensive care unit.
The resuscitation of the child with GI hemorrhage is directed by the magnitude of the bleeding. A history is obtained, and the location and magnitude of the bleeding is estimated. A history of medications that affect the coagulation system is elicited. A complete blood count, platelet count, liver function tests, and coagulation studies are obtained. The resuscitation itself is as one would conduct for a patient with hemorrhage from any site.
After the initial resuscitation, the management plan shifts to that of diagnosis and treatment. The following section presents a discussion of organ-specific sources of GI bleeding followed by an age-related approach to the diagnostic evaluation of children with upper and lower GI hemorrhage.
SOURCES OF UPPER GASTROINTESTINAL BLEEDING
Esophagitis
Esophagitis is responsible for 15% to 19% of upper GI hemorrhage found in childhood (1). In infancy, esophagitis
often results from gastroesophageal reflux (GER). Although biopsy evidence of esophagitis is present in 61% to 83% of infants with GER, clinically apparent bleeding is unusual (2). Esophagitis usually causes occult blood loss and anemia. Acute bleeding from esophagitis is treated with nasogastric decompression, histamine-2 (H2) antagonists, proton pump inhibitors, or oral antacids, and positional therapy. If nonoperative therapy is successful, reflux is documented, and medical management is instituted. If medical management does not control reflux, an antireflux operation is performed. Severity of esophagitis has not been shown to correlate with the need for antireflux surgery (3).
often results from gastroesophageal reflux (GER). Although biopsy evidence of esophagitis is present in 61% to 83% of infants with GER, clinically apparent bleeding is unusual (2). Esophagitis usually causes occult blood loss and anemia. Acute bleeding from esophagitis is treated with nasogastric decompression, histamine-2 (H2) antagonists, proton pump inhibitors, or oral antacids, and positional therapy. If nonoperative therapy is successful, reflux is documented, and medical management is instituted. If medical management does not control reflux, an antireflux operation is performed. Severity of esophagitis has not been shown to correlate with the need for antireflux surgery (3).
TABLE 71-1 Common Causes of Gastrointestinal Hemorrhage in Children. | |
|---|---|
|
Esophageal Varices
Variceal hemorrhage is an important cause of 7% to 10% of upper GI hemorrhage in children and adolescents (4). Most children with portal hypertension and variceal hemorrhage present before 5 years of age. Portal hypertension in children results from both extrahepatic and intrahepatic causes. Among the known causes of extrahepatic portal hypertension are neonatal omphalitis, umbilical vein catheterization, and portal vein hypoplasia associated with Klippel-Trenaunay syndrome. Intrahepatic portal hypertension most commonly results from cirrhosis secondary to biliary atresia. Nonoperative management includes endoscopic variceal ligation or injection, placement of a Sengkstaken-Blakemore tube, and use of intravenous octreotide or vasopressin (5). Operative management includes portosystemic shunts, variceal ligation, esophageal division, and esophageal devascularization.
Stress Ulceration and Gastritis
Gastritis is responsible for 13% to 22% of pediatric upper GI hemorrhage and may be defined as primary or secondary. Primary gastritis in children is most often due to Helicobacter pylori infection. This rarely results in symptomatic gastritis (6). Its importance lies in its relation to peptic ulcer disease in children. Secondary gastritis resulting in GI hemorrhage can occur secondary to mechanical injury to the gastric mucosa as occurs with long-term nasogastric suction, or due to stress ulceration of the stomach. Premature infants and children sustaining trauma, burns, or serious medical illnesses are at risk for developing stress ulcers. Stress ulceration has been found to be responsible for approximately 10% of GI hemorrhage in pediatric intensive care patients (7). The pathophysiology of stress ulceration of the stomach is not fully defined. Important concepts include loss of mucosal barrier function, alterations in gastric microcirculation, back diffusion of hydrogen ion, and duodenogastric bile reflux. Neonates and children present with either “coffee ground” emesis or frank hematemesis. Children with significant ongoing bleeding should undergo prompt diagnostic endoscopy. Initial nonoperative management includes transfusion, correction of any coagulopathy, and the use of antacids, H2-antagonists, proton pump inhibitors, or sucralfate. If correctable causes of stress exist, such as burn wound infection or intraabdominal abscess, they should be treated concurrently. If initial management does not succeed,
operative exploration should be performed, although this is uncommon in contemporary pediatric surgical practice.
operative exploration should be performed, although this is uncommon in contemporary pediatric surgical practice.
Preoperative endoscopy can identify bleeding sites in the stomach in most patients. The presence or absence of associated duodenal bleeding from either duodenitis or peptic ulcer disease should be established prospectively. In the absence of duodenal bleeding, the initial operative maneuver should be a gastrotomy and the walls of the stomach inspected. The least ablative surgery should be performed. If possible, superficial erosions should be oversewn. Focal ulcers, Dieulafoy lesions, or isolated areas of bleeding can be resected locally or with a standard partial gastrectomy, but this is rarely necessary. Problems arise in patients with life-threatening hemorrhage from multiple sites on the gastric wall. Gastric devascularization has been used successfully to treat this condition (8). If this fails to stop the bleeding, total gastrectomy is an option. It is difficult to identify the appropriate application of vagotomy in these situations, but it may be recommended in children with associated duodenal ulcer disease.
Having discussed the operative management of the child with stress gastritis, it is important to emphasize the importance of prophylaxis. Stressed children should have aggressive attempts to raise their gastric pH to above 4.5 with either H2-antagonists, proton pump inhibitors, or antacids. This is usually sufficient therapy.
Peptic Ulcer Disease
Peptic ulcer disease can afflict children in all age groups. The capacity of the stomach to produce acid is present in the premature and full-term newborn infant (9). About one-half of children with acute ulcers present with GI hemorrhage. Gastric and duodenal ulcer disease is responsible for 18% and 11% of upper GI hemorrhage, respectively. Fortunately, this bleeding is usually not life threatening. Endoscopy should accurately localize the ulcer. Endoscopic techniques, such as heater probe coagulation or bipolar cautery, may be used to arrest the bleeding focus. If this fails, operation is necessary. Standard therapy includes exposure of the ulcer and three-point suture ligation of the ulcer bed. Ligation of the gastroduodenal artery may be beneficial. Vagotomy and a drainage procedure do not appear necessary for acute bleeding ulcers in children. After successful medical or surgical management, children are placed on an H2-antagonist for 3 to 6 months. The Zollinger-Ellison syndrome should be considered in any child with multiple ulcers or recurrent ulcers. Serum gastrin levels are obtained to rule out this syndrome.
SOURCES OF LOWER GASTROINTESTINAL BLEEDING
Meckel’s Diverticulum
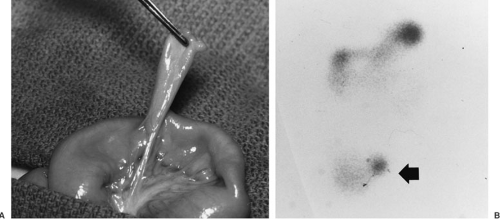
Lower GI hemorrhage is a common complication of a Meckel’s diverticulum. Most children affected are younger than 5 years of age (10). In 95% of cases, bleeding results from ulceration of adjacent ileal mucosa by heterotopic acid secreting gastric or pancreatic mucosa contained in the diverticulum. Bleeding is usually painless. The diagnostic test of choice is a technetium-99m pertechnetate scan (Fig. 71-1). Uptake of the isotope by the heterotopic mucosa allows identification of the bleeding source. The technetium scan is 85% sensitive and 95% specific for a Meckel’s diverticulum presenting with hemorrhage. Diagnostic yield of the scan can be increased by
pharmacologic intervention with H2-blockers to inhibit isotope excretion from ectopic gastric mucosa. In addition, pentagastrin stimulates isotope uptake by the ectopic mucosa and glucagon decreases peristalsis, thereby increasing isotope retention time within the diverticula (11). Nonbleeding Meckel’s diverticuli can also be diagnosed by visceral angiography (12).
pharmacologic intervention with H2-blockers to inhibit isotope excretion from ectopic gastric mucosa. In addition, pentagastrin stimulates isotope uptake by the ectopic mucosa and glucagon decreases peristalsis, thereby increasing isotope retention time within the diverticula (11). Nonbleeding Meckel’s diverticuli can also be diagnosed by visceral angiography (12).
After the child is stabilized, the diverticulum is excised surgically. Care is taken to assess the adjacent ileum for the presence of an ulcer that may need to be resected with the diverticulum. Many Meckel’s diverticuli are amenable to laparoscopic excision.
Intestinal Polyps
Juvenile Polyps
Juvenile polyps have an estimated prevalence of 1% to 2% in the general population. The most common presentation for a juvenile polyp is painless rectal bleeding, which occurs in 92% of these patients (13). Bleeding from juvenile polyps usually occurs in preschool-age children. These polyps, also known as hamartomatous or retention polyps, are benign, with less than 5% of polyps showing signs of dysplasia (14). Bleeding originates from the friable surface of the polyps. The cause of these polyps is unknown. The presence of interstitial eosinophils raises the possibility of an allergic reaction of the colonic mucosa. Recent application of pancolonoscopy has revealed that greater than 55% of polyps are found in the rectosigmoid colon, 15% in the descending colon, 15% in the transverse colon, and 15% in the right colon. Colonoscopy has refuted the assumption that juvenile polyps are usually solitary because multiple polyps are found in greater than 50% of patients presenting with a lower GI hemorrhage (15). This finding has taken on new significance, as the presence of three to five polyps is consistent with the premalignant condition juvenile polyposis coli (JPC) (16). Curative treatment by colonoscopic polypectomy or transanal excision is achieved in 97% of cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree