Gastroesophageal Reflux
Robert P. Foglia
Department of Surgery, Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, Missouri 63110.
Gastroesophageal reflux (GER) is a major cause of morbidity in children. In many children’s hospitals, related operations are one of the most common intraabdominal procedures performed. Its incidence is difficult to quantitate because the definition and evaluation vary among institutions. GER is a relatively newly described disease entity. Research in the 1960s generally ascribed GER to the presence of a partial thoracic stomach and identified a hiatal hernia as a prominent component of the pathophysiology. The 1960s and 1970s saw progressive improvement in diagnostic studies used to assess GER. The first large report of surgically treated infants with GER opened the modern era of evaluation and treatment of this entity in the pediatric population in 1974 (1).
ANATOMY AND PHYSIOLOGY
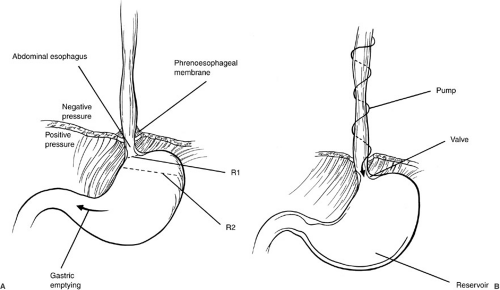
The anatomy and physiology of GER can be considered in terms of the dynamic events occurring in three distinct areas: the esophagus, the gastroesophageal (GE) junction, and the stomach (Fig. 66-1). The esophagus functions as a conduit to transport material from the pharynx to the stomach. The initial propulsion of food into the esophagus is under skeletal muscle control. After the bolus begins to descend in the esophagus, propulsion is due to smooth muscle function from the pharynx and the cricopharyngeus. In the normal circumstance, a coordinated primary esophageal stripping wave moves the food through the esophagus. A secondary esophageal wave serves to clear any residual material not moved distally by the primary wave, or any gastric contents that reflux into the esophagus. The propulsion of a bolus of food down the length of the esophagus can be considered as a pump function which allows the bolus to move from a negative pressure area (the thorax, with an average intrathoracic pressure of –6 torr to the stomach with an intraperitoneal pressure of +6 torr).
Normally, there is an intraabdominal segment of the distal esophagus below the diaphragm. Although there is not a true anatomic sphincter present, the lower esophageal segment at the level of the diaphragm functions as a physiologic sphincter. Factors contributing to the GE junction function include the length of the intraabdominal esophagus, the configuration of the muscle fibers at the GE junction, the relative difference in the diameters of the intraabdominal portion of the esophagus and the fundus of the stomach, and the presence of a high-pressure zone at the GE junction. Typically, this sphincterlike area remains closed until a peristaltic wave transfers a bolus of food to the GE junction, at which time the sphincter relaxes. After the peristaltic wave passes and the foodbolus traverse the area, the sphincter again closes (2). It appears that lower esophageal sphincter pressure (LESP) and relaxation at the GE junction are controlled by several mechanisms, both neural and hormonal.
The lower esophageal sphincter (LES) is regulated by the adrenergic anatomic nervous system. However, LESP is decreased with alpha blockers or beta-adrenergic stimulation. In addition, both excitatory and inhibitory impulses are conducted by the vagus nerves to the esophagus and the GE junction. Hormones such as gastrin and motilin increase LESP as do peptides such as bombesin, beta enkephalin, and substance P. In contrast, LESP is lowered by secretin, cholecystokinin, glucagon, somatostatin, and estrogen. Likewise, mediators such as vasoactive intestinal peptide, neuropeptide Y, and gastric inhibitory peptide all decrease LESP. Pharmacologic agents that increase LESP include antacids, metoclopramide, cholinergics, domperidone, and prostaglandin F. Other agents such as barbiturates, narcotics, benzodiazepines, theophylline, calcium channel blockers, caffeine, alcohol, and prostaglandin E all lower LESP.
The stomach acts a reservoir, and the competency of the GE junction prevents pathologic reflux. If there is an obstruction to gastric emptying, either from a true mechanical problem or a physiologic abnormality, the contents
remain in the stomach for a protracted period, and intragastric pressure increases within the stomach in an attempt to more forcefully empty the gastric contents into the duodenum. However, this contraction and its force is not directed solely toward the gastric outlet. If there is concomitant dysfunction at the GE junction, acid reflux can occur.
remain in the stomach for a protracted period, and intragastric pressure increases within the stomach in an attempt to more forcefully empty the gastric contents into the duodenum. However, this contraction and its force is not directed solely toward the gastric outlet. If there is concomitant dysfunction at the GE junction, acid reflux can occur.
PATHOPHYSIOLOGY
Initially, pathologic GER was believed to result from a hiatal hernia and this was described as a “partial thoracic stomach”. In adult screening tests, about one-half of people 50 years of age have a radiographically demonstrable hiatal hernia. Most of these individuals do not have significant symptoms of GER. In children with pH probe-proven pathologic GER, the incidence of a hiatal hernia is 3% to 6%.
GER may be of marked significance or of little or no functional consequence. If a patient has brief episodes of acid reflux into the lower esophagus, but has no clinical symptoms, this is considered physiologic. An understanding of the elements that allow for a greater degree and amount of GER helps to explain both the process and the strategies that can be used to correct the reflux (Fig. 66-1). The normal position of an intraabdominal segment of esophagus allows for the development of a high-pressure zone. The acute angle (angle of His) between the intraabdominal portion of the esophagus and the cephalad end of the greater curvature of the stomach allows for a marked difference in the diameter of the intraabdominal portion of the esophagus and the superior portion of the stomach. The law of LaPlace states that the tension in two areas that are in continuity is inversely related to their radii. Thus, if the diameter (or radius) of the stomach at the level of the fundus (R2) is five times longer than the diameter (or radius) of the intraabdominal portion of the esophagus (R1, Fig. 66-1), then the pressure is five times greater in the esophagus than in the stomach, and this tends to prevent reflux. If the angle of His is not present, the relation of the distal esophagus and the proximal stomach becomes more like the shape of a funnel. The difference in the esophageal and gastric diameters is smaller, and the pressure differential between the two areas is less, affording greater potential for reflux. This problem is seen in patients with hiatal hernias, in whom a portion of the stomach is above the diaphragm and the angle of His may be absent.
In the evaluation of a patient with suspected GER, it is important to identify whether the child is receiving
any medication that might adversely alter LESP. A classic situation is a premature infant who is receiving theophylline as treatment for apnea and bradycardia. The medication is given to increase the respiratory drive; however, the apnea and bradycardia might result from silent reflux, and the theophylline may actually exacerbatè GER. Conversely, metoclopramide both increases LESP and acts as a prokinetic agent. These and other drugs or stimulation of other hormones can modulate GER.
any medication that might adversely alter LESP. A classic situation is a premature infant who is receiving theophylline as treatment for apnea and bradycardia. The medication is given to increase the respiratory drive; however, the apnea and bradycardia might result from silent reflux, and the theophylline may actually exacerbatè GER. Conversely, metoclopramide both increases LESP and acts as a prokinetic agent. These and other drugs or stimulation of other hormones can modulate GER.
As noted previously, the esophagus can be considered to be a type of pump that moves liquids and solids into the stomach. In the semiupright or upright position, esophageal motility and gravity transport food down the esophagus into the stomach and clear the esophagus of refluxed material (Fig. 66-1B). The LES and the GE junction function as a physiologic valve and depend on an intraabdominal length of esophagus and a high-pressure zone. The LES opens in response to an esophageal peristaltic wave and then closes afterward. In the pathologic circumstance, LESP decreases in the absence of a peristaltic wave and the GE junction opens. This allows gastric contents to reflux into the esophagus. The GE junction is highly influenced by medications and inflammation. A mechanically inadequate lower esophageal sphincter can result from inadequate sphincter pressure, inadequate intraabdominal esophageal length, or decreased abdominal pressure (3). The final component of this mechanical model is the stomach, which functions as a reservoir. Gastric abnormalities that can result in GER include increased gastric pressure caused by decreased or delayed gastric emptying, and increased acid secretions. Likewise, gastric dilation and retching can alter the anatomy in the area of the cardia and fundus, and afford greater likelihood of reflux. Note that, as the stomach fills and becomes distended, the upper portion of the stomach distends, and this may decrease the overall length of the high-pressure zone at the GE junction. In addition, reflux of duodenal contents into the stomach and subsequent duodenogastric reflux may affect the distal esophagus and the GE junction and cause more pathology than acid reflux alone. In summary, the antireflux barrier at the GE junction is a function of three components: (1) an adequate LESP, (2) adequate length of the LES, and (3) appropriate portion of intraabdominal esophagus subjected to positive abdominal pressure.
In some patients, GER is a component of a foregut motility disorder and can be associated with dyscoordinate esophageal peristalsis and motility, as well as delayed gastric emptying. GER can be quantitated by the number of times that acid is noted in the esophagus on a pH probe study. Fifteen to 20 brief episodes of incompetency of the GE junction occur normally daily (referred to as eructation or burping). If there is prompt esophageal clearance, this is not of functional significance. The combination of acid reflux and poor esophageal motility causes the esophagus to be bathed in acid for a protracted period. This inflammation serves to decrease LESP, and this leads to a vicious cycle of potentiating GER. Patients with associated tracheoesophageal fistulas often have dyscoordinate esophageal peristalsis; thus, they are at high risk for the development of pathologic acid reflux. These children can develop an anastomotic stricture due to technical problems with the anastomosis, such as tension and a marginal blood supply, combined with the subsequent effect of acid at the anastomosis. They also are at risk for esophagitis and stricture formation in the distal esophagus.
Strategies to correct GER should address abnormalities of the esophageal motility, ineffective LESP due to anatomic or pharmacologic causes, and gastric anomalies, especially delayed gastric emptying.
Reflux
With regard to the esophagus, the functional consequences of GER are the result of the refluxed material from the stomach. Previously, if was believed that the acidic pH of the gastric contents caused irritation of the esophageal mucosa leading to inflammation and pain, and manifested by esophagitis. Over a period of time, there could be associated bleeding and stricture formation. It is now known that acid reflux alone causes only mild esophageal mucosal damage. However, if the esophagus is exposed to both acid and pepsin, there will be a significant mucosal injury (4). Similarly, refluxed duodenal contents alone do not cause a significant esophageal injury, whereas duodenal contents and gastric contents combined cause a marked esophageal mucosal injury (5). The interplay of refluxed gastric and duodenal contents can have a mild or profound effect in regard to esophageal injury. For example, duodenal reflux can blunt peptic esophagitis in a patient in whom the acid inactivates trypsin, and the bile salts modulate pepsin. Conversely, if a patient refluxes duodenal contents into the stomach with a limited degree of acidity present, the net result would be the reflux of an alkaline bolus of duodenal and gastric contents into the esophagus. Thus, trypsin’s activity would be optimized, resulting in a greater degree of esophageal injury. One can see that hypothetically lowering the gastric pH might be beneficial in treating GER alone, but that it could be detrimental in the child with concomitant GER and duodenal reflux.
PRESENTATION
GER is defined by a lack of competency of the GE junction, allowing gastric contents to ascent into the esophagus. Virtually all humans have this occur regularly; the clinical objective is to differentiate those individuals with pathologic GER who are at risk for complications related
to this event. Presentation of pathologic GER vary, and not all children with reflux have emesis. Clinically significant GER can be categorized in three broad patterns of presentation. The first is characterized by overt emesis. The other two presentations are referred to as silent reflux because emesis is absent. The second involves reflux to the level of the epiglottis, with gastric contents spilling into the tracheobronchial tree and causing acid aspiration with respiratory symptoms. The third type of reflux involves the esophagus alone.
to this event. Presentation of pathologic GER vary, and not all children with reflux have emesis. Clinically significant GER can be categorized in three broad patterns of presentation. The first is characterized by overt emesis. The other two presentations are referred to as silent reflux because emesis is absent. The second involves reflux to the level of the epiglottis, with gastric contents spilling into the tracheobronchial tree and causing acid aspiration with respiratory symptoms. The third type of reflux involves the esophagus alone.
Infants normally have some degree of emesis, ranging from a wet burp to regurgitation of a significant amount, if not all, of a recent feeding. Children with pathologic GER, however, often regurgitate large volumes more frequently. The differential diagnosis of children with emesis should take into account the character of the emesis. Bilious emesis in young children is considered to be caused by obstruction until proved otherwise. The emesis with GER is nonbilious and typically occurs during a feeding or shortly after a feeding. Often, the parent finds the crib sheet or pillow stained by the vomited material. In the child who is several weeks of age, the major differential diagnosis is pyloric stenosis. If the GER is severe, weight loss and failure to thrive can occur. Parents may describe the number of times the child’s clothes must be changed each day. In older children, the repeated emesis can lead to social problems and poor self-esteem. The presence of emesis makes the diagnosis of reflux easy to identify; however, the lack of emesis does not rule out GER.
Aspiration of gastric contents into the tracheobronchial tree can cause apnea, pneumonia, bronchitis, and asthma (6). Gastric contents can be a potent trigger for apnea and bradycardia from reflex laryngospasm. A decrease in esophageal pH can be associated with the simultaneous development of respiratory symptoms. These respiratory symptoms can be as mild as coughing or as critical as apnea, which can be a mechanism for sudden infant death syndrome (7). In infants and children with recurrent episodes of pneumonia believed to be aspiration related, the association with GER may be relatively straightforward. Conversely, a number of patients with reflux may have asthma or bronchitis for many years, and this may be the primary or even sole symptom of the GER. Although the concept of reflux causing pulmonary symptoms from aspiration has been well known, there and now data which show that exposure of the distal esophagus to acid is associated with reflex bronchocon-striction (8).
Symptoms related solely to acid bathing the esophagus include poor feeding, dysphagia, and Sandifer syndrome. In the older child, there may be a complaint of heartburn. Findings may include esophagitis, esophageal ulceration, stricture formation, melena, bleeding, anemia, and Barrett’s esophagus. A symptom constellation may be relatively acute or chronic in regard to its presentation.
ASSOCIATED ANOMALIES
Children with esophageal atresia are likely to have reflux for several reasons. Because of the discontinuity of the esophagus, there is a lack of the normal innervation into the area of the LES. In addition, at the time of repair of esophageal atresia, there may be a need to mobilize the distal esophageal segment to achieve sufficient length to carry out an anastomosis without tension. This can change the configuration of the angle of His, thereby disrupting one anatomic antireflux mechanism. Extensive mobilization of the distal esophagus can actually pull part of the stomach up through the diaphragm, decreasing or eliminating the intraabdominal portion of the esophagus and creating a hiatal hernia. This may be true in the typical tracheoesophageal fistula repair with the distal esophageal segment attached to the trachea, and is of even more concern in children with pure esophageal atresia and a long gap between the two esophageal segments. If GER is present, its sequelae may be more severe in these patients because of esophageal dysmotility and ineffective acid clearance from the distal esophagus. In this event, the gastric acid contents remain in contact with the esophagus longer, causing further inflammation of the distal esophagus.
After repair of a diaphragmatic hernia, patients are more likely to have pathologic GER, with an incidence as high as 35% (9,10). Closure of the diaphragmatic defect can place tension on the esophageal crura, pulling the left crus laterally and altering the anatomy of the GE junction. Hiatal hernia is uncommon in children with GER, but when present, this confers a high likelihood that the reflux will not respond to medical therapy. Finally, there is a high incidence of GER in neurologically impaired patients. It is unclear whether this is due to a central mechanism or whether chronic retching in some of these children causes reflux.
DIAGNOSIS
The evaluation of the infant or child with suspected pathologic GER begins with a detailed history and physical examination. In the infant, pyloric stenosis and GER can be confused. The pattern of emesis, both in terms of frequency and severity, often allows for differentiation. Emesis with GER is typically nonbilious. Bilious emesis should be considered in the young child to be due to malrotation until proved otherwise (11). The patient may have a history of recurrent upper respiratory infections, bronchitis, pneumonitis, pneumonia, or asthma. Any of these can be indicative of GER. Poor feeding can be due to repetitive bouts of reflux and subsequent esophagitis or esophageal stricture formation.
Physical examination can reveal obvious emesis, a cough related to GER, pneumonia, or Sandifer syndrome. In the latter circumstance, the infant repetitively turns his
or her head to one side to improve peristalsis in the esophagus. This can be confused with torticollis. It is associated with neck extension and arching of the back, presumably the consequences of esophagitis induced pain. In some patients, a period of hospitalization may ascertain whether there is a significant feeding problem. Specific questions about the amount of food the infant is taking can help define failure to thrive and potential causes of emesis. A period of observation also allows for identification of the frequency and severity of GER, as well as assessment of the effect of treatment strategies.
or her head to one side to improve peristalsis in the esophagus. This can be confused with torticollis. It is associated with neck extension and arching of the back, presumably the consequences of esophagitis induced pain. In some patients, a period of hospitalization may ascertain whether there is a significant feeding problem. Specific questions about the amount of food the infant is taking can help define failure to thrive and potential causes of emesis. A period of observation also allows for identification of the frequency and severity of GER, as well as assessment of the effect of treatment strategies.
The diagnostic tests available to evaluate the patient with suspected GER include an esophagogram, esophageal pH probe measurement, scintigraphy, esophageal manometry, esophagoscopy, and biopsy. Each test has advantages and disadvantages, and is not performed in every patient.
Esophagogram
The esophagogram is the most commonly used diagnostic test in the evaluation of GER. It is an good as a screening test, is arguably the easiest test to perform, and provides information about the anatomy of the esophagus, stomach, and duodenum, as well as their respective function. Fluoroscopy can identify the presence of GER and whether the refluxed material remains in the esophagus alone or results in aspiration of acid into the tracheobronchial tree. Abnormalities of the esophagus, such as esophagitis, ulceration, stricture, and dilation, are identified (Figs. 66-2 and 66-3). The presence of normal peristalsis in the esophagus and stomach can be evaluated. The presence of a hiatal hernia, the position of the GE junction, the configuration of the stomach, and the angle of His can also be evaluated. In addition, a number of abnormalities that cause a delay in gastric emptying, such as pyloric stenosis, malrotation, or a duodenal web, can be identified. The esophagogram is reviewed with spot films and cinefluoroscopy. The latter gives a representation of the dynamic process of swallowing and GER. Another point of evaluation in this study is the rapidity of esophageal clearance of refluxed gastric contents. In the esophagus with poor peristalsis, refluxed contrast may remain for a lengthy period of time. In the patient who has had repair of esophageal atresia, the esophagogram can identify abnormal esophageal motility, an anastomotic stricture, a more distal stricture, and GER. The overall sensitivity of the esophagogram in the evaluation of GER is about 85% (12). A major limitation is the short interval used to evaluate the subject. If reflux is noted during the deglutition of barium, or if it is noted when the stomach is filled with contrast, a positive diagnosis is made. The absence of reflux during the relatively short evaluation, however, does not rule out its presence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree