The fetus depends almost exclusively on the placenta for nutritional, respiratory, and excretory functions. The placenta, growing steadily as gestation progresses, parallels fetal growth. Studies of placental growth and physiology in disease states suggest that placental growth and size are determined by the fetus and modulated by maternal factors. Normal placental-to-fetal weight ratios are approximately 1:6. As the placenta grows, villous processes increase in number as fetal vasculature expands, and by the third trimester, a large surface area is available to the maternal and fetal circulations.
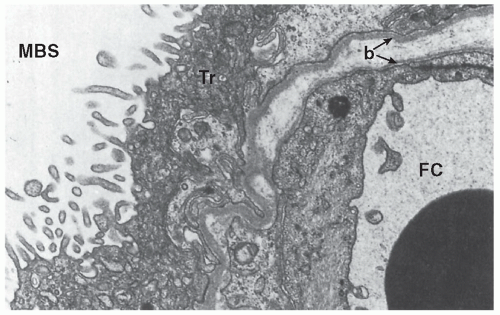
Most placental transport is transcellular. Although the placenta often is thought of as a “separating membrane,” it is actually a series of membranes. The most efficient areas for maternofetal exchange are the epithelial plates, which consist of thinly stretched, attenuated villous tissue separating maternal blood in the intervillous space from fetal blood in the fetal sinusoids. To cross epithelial plates from the maternal to the fetal side, a substance must traverse:
Simple Diffusion
Many nutrients, metabolites, and excretory products cross the placenta by diffusion. Diffusion of substances in the placenta depends on multiple factors, as summarized in
Table 11.1.
The amount of a nutrient delivered to the placenta is directly proportional to its concentration in the maternal bloodstream, which depends on nutritional intake and gastrointestinal absorption. Famine, maternal gastrointestinal diseases that interfere with absorption, or maternal pulmonary diseases that interfere with alveolar exchange may significantly affect blood concentrations and the transfer and accrual of fetal fuels. A lack of fetal fuels produces fetal and placental growth restriction. Abnormalities in maternal homeostatic mechanisms may produce either an insufficiency or an abundance of nutrients. For example, in poorly controlled diabetes, maternal hyperglycemia, hyperaminoacidemia, and hypertriglyceridemia allow unrestrained nutrient delivery to the fetus with excessive growth of fetal organs, body fat, and the placenta (
3).
Delivery of a nutrient to the placenta is directly proportional to blood flow in the intervillous space. Maternal blood volume gradually increases to 30% to 40% higher than prepregnancy volume, with 40% directed to the uterus and placenta. Maternal cardiac disease with lower cardiac output may result in fetal and placental growth restriction. Even in healthy women, maternal position influences blood flow to the uterus. Normal pregnant women have an 18% lower cardiac output in the standing position compared with lying on their sides, perhaps explaining why women who stand at work throughout their pregnancy have newborns of lower birth weight.
Fetal factors influencing diffusion are those that affect nutrient delivery to the fetal side of the placenta. The concentration of a substance in the umbilical artery depends on the amount of prior placental transfer, absorption from swallowed amniotic fluid, and
fetal metabolism. Fetal blood flow to the uterus depends on fetal cardiac output and placental vascular tone. Normally, fetal vessels on the chorionic plate are maximally dilated, providing the least resistance to flow.
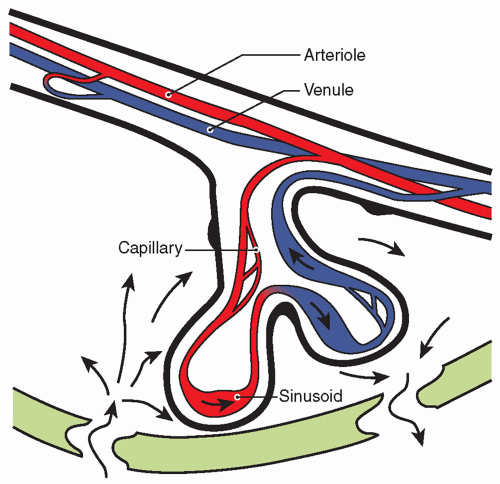
Numerous placental factors influence diffusion. Overall, transfer is governed by the quantity of epithelial plates, which are specialized regions of enhanced diffusion where the interhemal barrier is less than a few micrometers. The human placenta has an intervillous pool flow system in which fetal capillaries in terminal villi are bathed in a maternal blood reservoir continuously filled by arteries and drained by veins (
Fig. 11.3). Concurrent and countercurrent flows exist in areas of uneven distribution of flow (i.e., shunting), where a portion of the villus is well supplied by maternal blood but poorly supplied by fetal blood; in other areas, the opposite occurs.
Stereochemical characteristics of a substance are major factors in transferability. Small, compact, nonpolar, lipophilic substances are transferred most efficiently. The placenta is relatively impermeable to large, polar molecules that do not have specific transport systems or carrier proteins or are unable to take advantage of an analogous transport system to aid in their transfer. For example, α-fetoprotein (AFP), a 70-kd fetal protein, does not transfer to the maternal side in appreciable amounts despite large quantities in fetal blood. Maternal AFP is derived from transplacental transfer from fetal blood and transmembrane (i.e., chorioamnion) transfer from amniotic fluid. The fetal blood AFP level at 17 weeks of gestation is approximately 3 mg/mL when the maternal blood level is approximately 0.1 mg/mL, resulting in a fetomaternal gradient of approximately 30,000:1. The low level of fetomaternal transplacental transfer allows detection of elevated maternal serum levels from transmembrane (i.e., amniochorion) transfer of abnormally high amniotic fluid AFP, which provides the basis of maternal serum AFP screening for neural tube defects. False-positive elevations of maternal serum AFP (i.e., high maternal serum value with a structurally normal fetus) suggest placental microabruptions, or loss of integrity of the maternofetal barrier, and forecast a higher rate of fetal morbidity.
Membrane characteristics regulate transport. The fluidity of the membrane, determined by the degree and character of membraneincorporated phospholipids, influences transfer of certain substances. Diseases, such as diabetes, may influence membrane fluidity (
4).
The major driving force in favor of transfer by diffusion is the concentration gradient across the placenta; the resistance to diffusion is dictated by the nature of the molecule. The principles of diffusion of molecules that are generally applicable to biologic membranes hold true in the placenta, although specifics remain to be defined. Availability of a substance for diffusional transfer across the placenta is not always related to blood levels of that substance, because many metabolites, nutrients, and drugs that are poorly water soluble are protein bound.
Although proteins aid in delivery of these substances to the placenta, they may actually hinder transfer of the substances. It is the free, unbound—or soluble—fraction of a substance that is available for transfer. Conversely, high-affinity carrier proteins on the receiving side of the placenta drive diffusional transfer to their side by decreasing a ligand’s free fraction and increasing its maternofetal gradient. Oxygen, for example, is 98% bound to hemoglobin. It is the transplacental difference in the partial pressure of dissolved oxygen (PO
2) that determines the diffusion pressure. The more oxygen-avid fetal hemoglobin counterbalances the resistance to transfer from the maternal circulation. The O
2 content (i.e., dissolved and hemoglobin-bound O
2) of the blood on each side of the placental membrane is determined principally by different affinities of maternal and fetal hemoglobin for oxygen. In humans, the fetal oxyhemoglobin dissociation curve is displaced leftward of the maternal curve, facilitating a much greater uptake of oxygen by fetal blood at the placental capillary level than would be possible otherwise. At any given PO
2, a much higher O
2 content is achieved in fetal blood than in maternal blood. The O
2 content in the umbilical vein (14.5 mL/dL) is as high as that of the uterine artery (15.8 mL/dL), despite an umbilical venous PO
2 of only 27 mm Hg (
Table 11.2). Relatively high fetal blood O
2 content confers on the fetus the ability to deliver sufficient oxygen to peripheral tissue despite low PO
2. Low PO
2 may be essential to fetal physiologic adaptation to maintain high pulmonary vascular resistance and to keep the ductus arteriosus open.
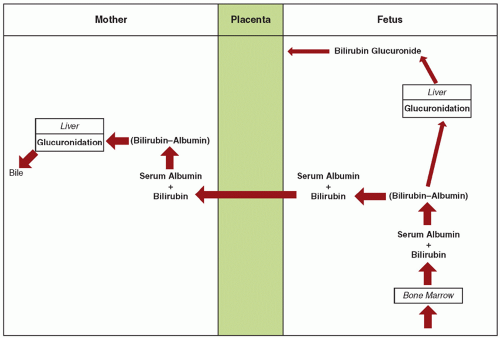
The excretion of bilirubin provides an example of fetomaternal interaction using specific permeability properties of the placenta to accomplish a given objective (
5). Before birth, elimination of bilirubin from the fetus is by diffusional transfer through the placenta to the mother. The placenta is extremely permeable to unconjugated bilirubin but relatively impermeable to bilirubin glucuronide (i.e., conjugated bilirubin). In the fetus, because of minimal bilirubin glucuronyltransferase, hepatic conjugation of bilirubin is suppressed. Because fetal bilirubin is predominantly unconjugated and highly lipid soluble, it diffuses freely from the fetal to the maternal side. After transfer to the mother, it is efficiently conjugated and excreted (
Fig. 11.4).
Active Transport
To provide appropriate fuels for fetal growth, specific energy-requiring transport mechanisms in the microvillous surface aid in transfer of substances that are not readily lipid soluble and are required in large amounts by the fetus.
Most amino acids cross the placenta by an active transport mechanism (
9,
10,
11). Active amino acid uptake has two major purposes: transfer to the fetus and placental production of peptide hormones.
Transfer from maternal to fetal circulation is especially important for the essential amino acids required for fetal growth, including the essential adult amino acids histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine and the proposed fetal essential amino acids cysteine, tyrosine, histidine, and taurine. Early in development, before maturation of fetal metabolic systems, all amino acids are essential to the fetus. Fetal amino acid levels are 1.5- to 5-fold higher than are maternal levels, confirming a transport process against a concentration gradient.
Placental transfer of amino acids is stereospecific, with the natural L-form preferred. Transport of amino acids by animal cells is mediated by specific carrier systems that have overlapping substrate reactivities. In human villous tissue fragments, three carrier systems exist for neutral amino acids (
12).
System A: sodium dependent, reversible at low pH, most reactive with short, polar, or linear side chain amino acids (e.g., alanine, glycine).
System L: sodium independent, most reactive with large, apolar, branched-chain, and aromatic amino acids (e.g., leucine, isoleucine, tyrosine, tryptophan, valine, phenylalanine, methionine, glutamine).
ASC system: sodium dependent, involved in transport of alanine, serine, and cysteine (ASC).
B system: most likely exists in placenta for taurine transport (
13). Taurine, although produced by the maternal liver from cysteine and methionine, is essential for fetal neurologic development but is not produced by the fetus.
Certain drugs cross the placenta by active transport. Zidovudine, used for treating HIV, has been found in the perfused human
placental model to cross from the maternal to the fetal side by energy-dependent, active transport (
14). Because zidovudine is a thymidine analog, it may take advantage of placental thymidine transport systems. Zidovudine levels are higher in cord blood than in maternal blood, suggesting transport against a concentration gradient and an active transport mechanism.
Receptor-mediated Endocytosis
Although many large protein molecules cross the placenta by pinocytosis in extremely small quantities, specific receptor-mediated processes expedite transfer of certain larger substances that are required by the fetus. The receptor-rich microvillous brush border of the syncytiotrophoblast and the numerous coated micropinocytotic vesicles found just beneath it provide anatomic evidence for receptor-mediated endocytosis (
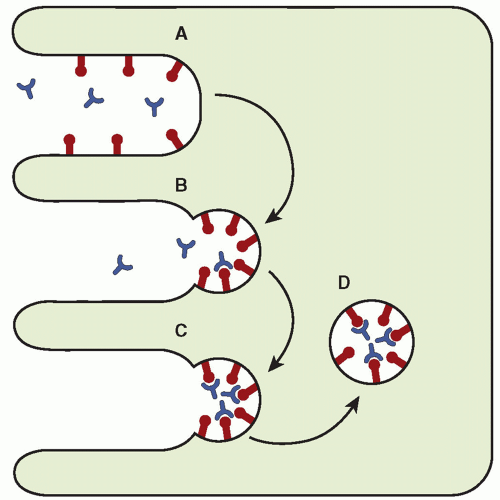
15). The receptors involved in this process, found on the surface of the syncytiotrophoblast, are thought to extend through the glycocalyx layer of the cell membrane and bind to the protein clathrin to form a membrane complex. After the ligands are bound to their receptors, aggregation and internalization occur to form a cytoplasmic-coated vesicle (
Fig. 11.5). Destiny of the contents of the vesicles depends on the ligand.
Maternal immunoglobulin (Ig) molecules are transferred to the fetus by receptor-mediated endocytosis. IgG subclasses 1 and 3 and IgA are known to cross the placenta. Once internalized, the intact Ig molecules within the vesicles are delivered from the cytoplasm of syncytiotrophoblast through the capillary endothelial cell and into the fetal circulation (
16,
17). Antenatal fetal transfer of maternal IgG antibodies may interfere with antibody-based diagnostic testing in the fetus, necessitating analysis of the fetal-specific IgM antibody. Developmentally, the transfer of maternal IgG to the fetus is probably protective and beneficial, but this transfer backfires in some situations, such as immune fetal hydrops (i.e., erythroblastosis fetalis) and alloimmune fetal thrombocytopenia. By receptor-mediated endocytosis, anti-D or another blood group antibody crosses the placenta to cause fetal hemolytic anemia, and anti-Pl
A1 crosses the placenta to cause fetal thrombocytopenia (
18).
Transfer of transferrin-iron complex into the placental syncytiotrophoblast occurs through receptor-mediated endocytosis. Brush border membrane transferrin-specific receptors on the maternal side of the syncytiotrophoblast bind transferrin-iron complex, and aggregate and internalize it to form vesicles of transferrin-iron
complexes. In the cytoplasm, the complexes dissociate to form apotransferrin and ferrous iron. Apotransferrin is recycled to the maternal circulation, and ferrous iron is stored transiently as ferritin and released to the fetal circulation to be made into a complex with fetal transferrin. No maternal transferrin or placental ferritin is transferred to the fetus (
19). Maternofetal iron transport is independent of maternal levels.
Uptake of low-density lipoprotein (LDL) cholesterol from maternal blood for progesterone synthesis by the placental trophoblast is accomplished through receptor-mediated endocytosis. Specific receptors that have a high affinity for LDL but not for high-density lipoprotein (HDL) are located on the microvillous brush border of the syncytiotrophoblast. LDL binds to its receptor and is actively internalized. Within the cytoplasm, LDL vesicles fuse with lysosomes, where enzyme hydrolysis of cholesterol esters releases cholesterol for mitochondrial synthesis of progesterone.