● EBSTEIN ANOMALY
Definition, Spectrum of Disease, and Incidence
As explained in Chapters 5 and 7, the tricuspid valve inserts in the normal heart slightly more apically on the interventricular septum than the mitral valve. Ebstein anomaly belongs to the few abnormalities affecting the valve attachment. In this disease, the septal and posterior leaflets of the tricuspid valve are displaced inferiorly from the tricuspid valve annulus, toward the apex of the heart, and originate from the right ventricular myocardium (Figs. 20.1 and 20.2). The anterior tricuspid leaflet maintains its normal attachment to the tricuspid valve annulus. The proximal portion of the right ventricle is then continuous with the true right atrium and forms an “atrialized” portion of the right ventricle (Figs. 20.1 and 20.2). The spectrum of Ebstein anomaly is wide and varies from a minor form, with minimal displacement of the tricuspid valves with mild tricuspid regurgitation, to a severe form, with the “atrialization” of the entire right ventricle (Figs. 20.3 and 20.4).
Associated anomalies are not uncommon and include right ventricular outflow tract obstruction, either as pulmonary stenosis (Fig. 20.5) or atresia, and atrial and ventricular septal defects. The pathogenesis of pulmonary stenosis or atresia in association with Ebstein anomaly may be related to a reduction in flow across the pulmonary valve due to severe tricuspid regurgitation. Atrial septal defect may also result from the dilation of the right atrium due to the severe regurgitation noted in utero. Ebstein anomaly is one of the less common cardiac abnormalities occurring in about 0.5% to 1% of congenital heart disease in live births (1), with an equal male-to-female distribution (2). Ebstein anomaly is more common in prenatal series as it accounts for 3% to 7% of congenital heart disease in fetuses (3, 4). This higher prenatal rate is related to an increase in fetal or early neonatal death in severe cases due to severe tricuspid regurgitation and associated pulmonary hypoplasia.
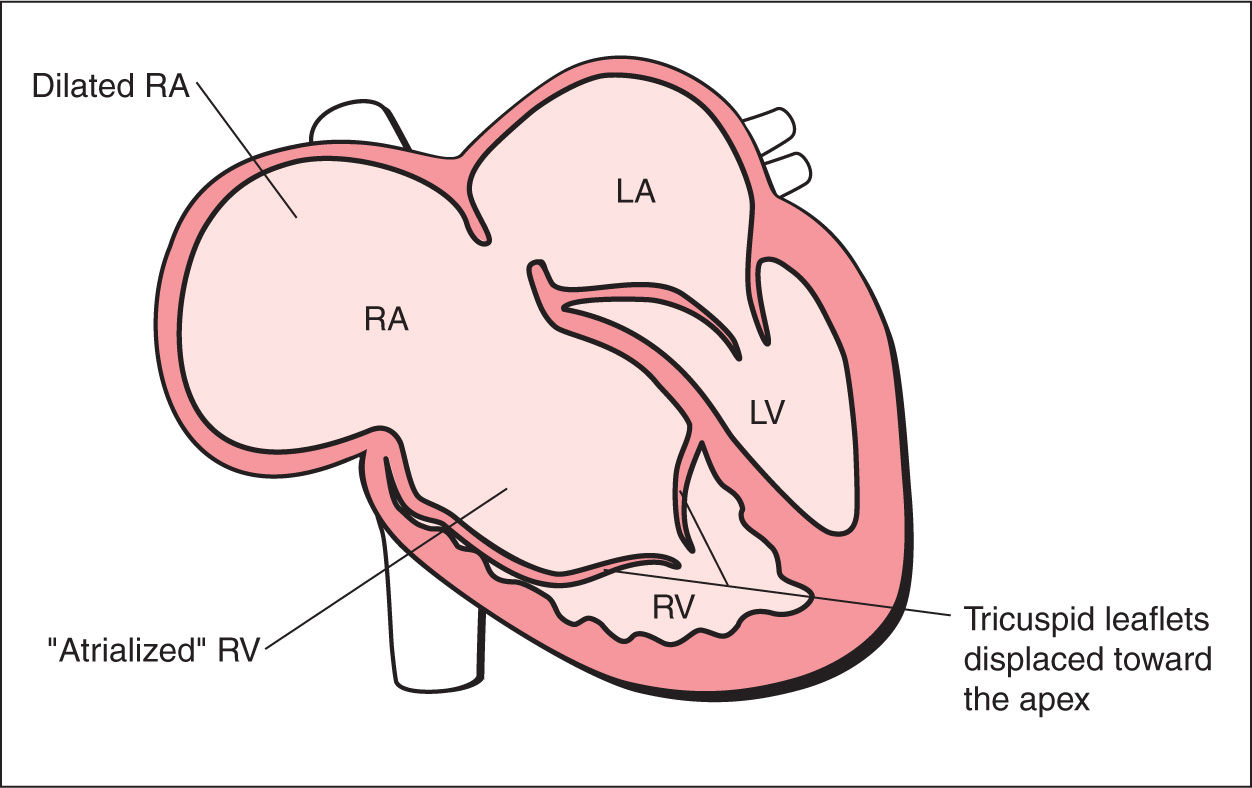
Figure 20.1: Schematic drawing of Ebstein anomaly. See text for details. LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle.
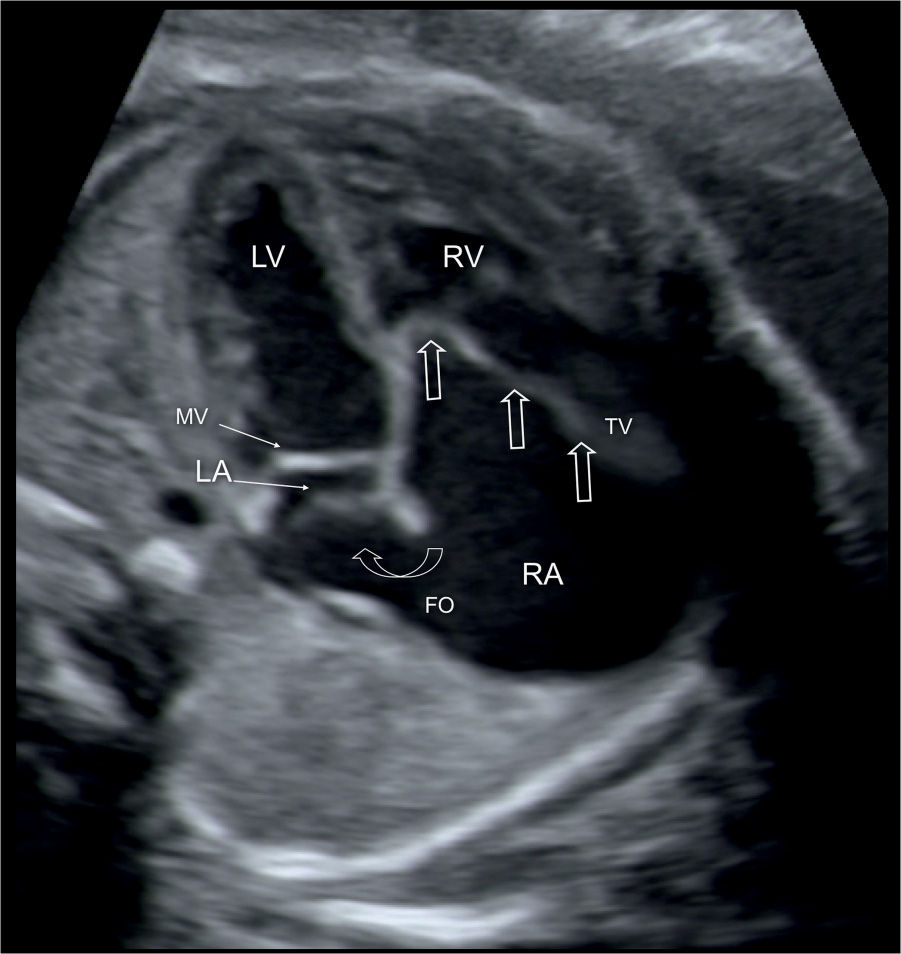
Figure 20.2: Apical four-chamber view in a fetus with Ebstein anomaly demonstrating the typical apical displacement of the tricuspid valve (TV) (open straight arrows) compared to the mitral valve (MV). The right atrium (RA) is dilated due to severe TV regurgitation, and the interatrial communication through the foramen ovale (FO) is wide (open curved arrow) due to increased right-to-left shunting of blood. LA, left atrium; LV, left ventricle; RV, right ventricle.
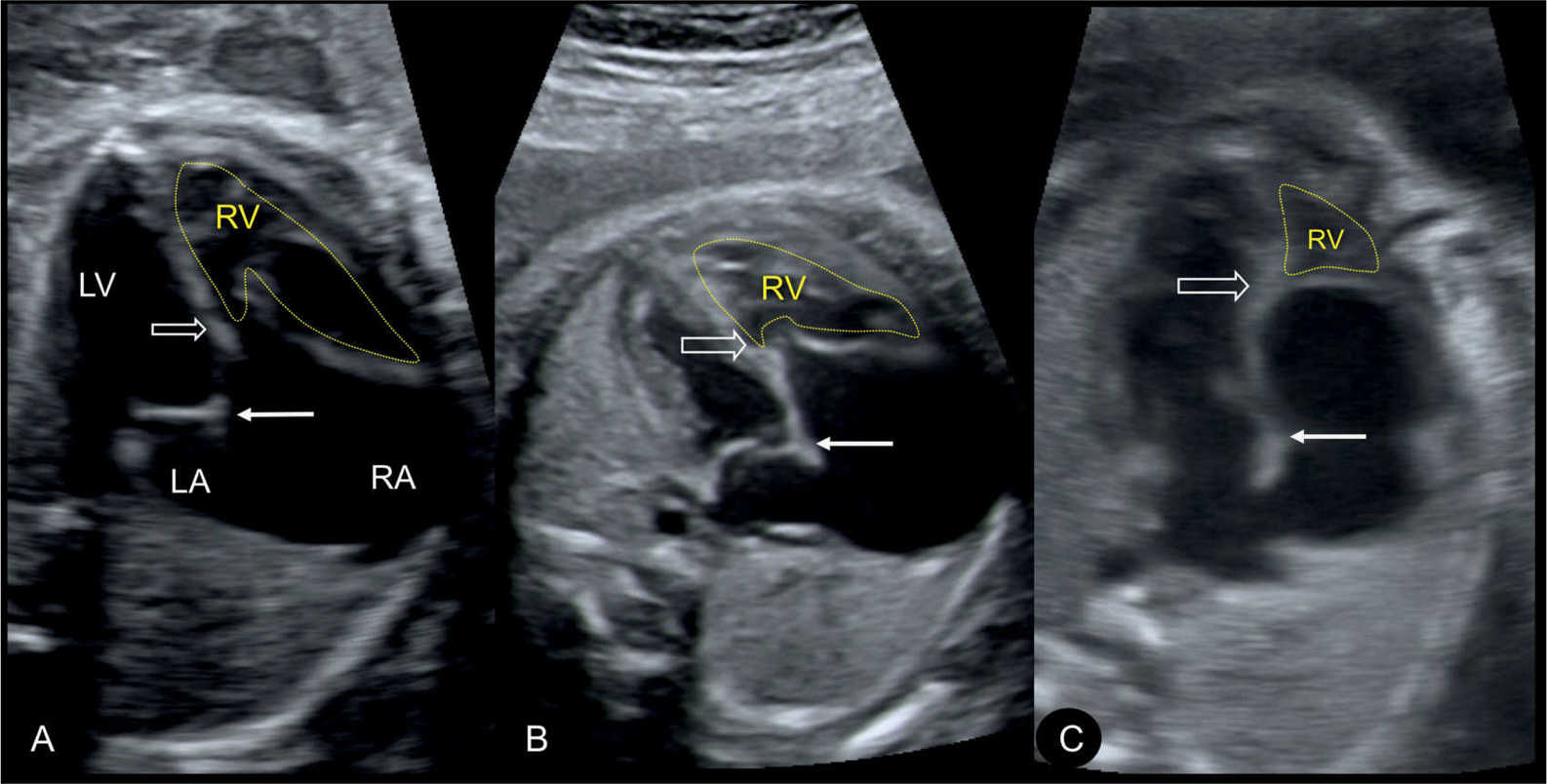
Figure 20.3: Apical four-chamber views in three fetuses (A–C) demonstrating the spectrum of disease in Ebstein anomaly. Septal attachment and displacement of the tricuspid valve differs as shown in A–C (open arrow). Location of mitral valve is shown by solid arrow. The atrialized portion of the right ventricle (RV) is biggest with more apical tricuspid valve displacement (C). LA, left atrium; LV, left ventricle; RA, right atrium.
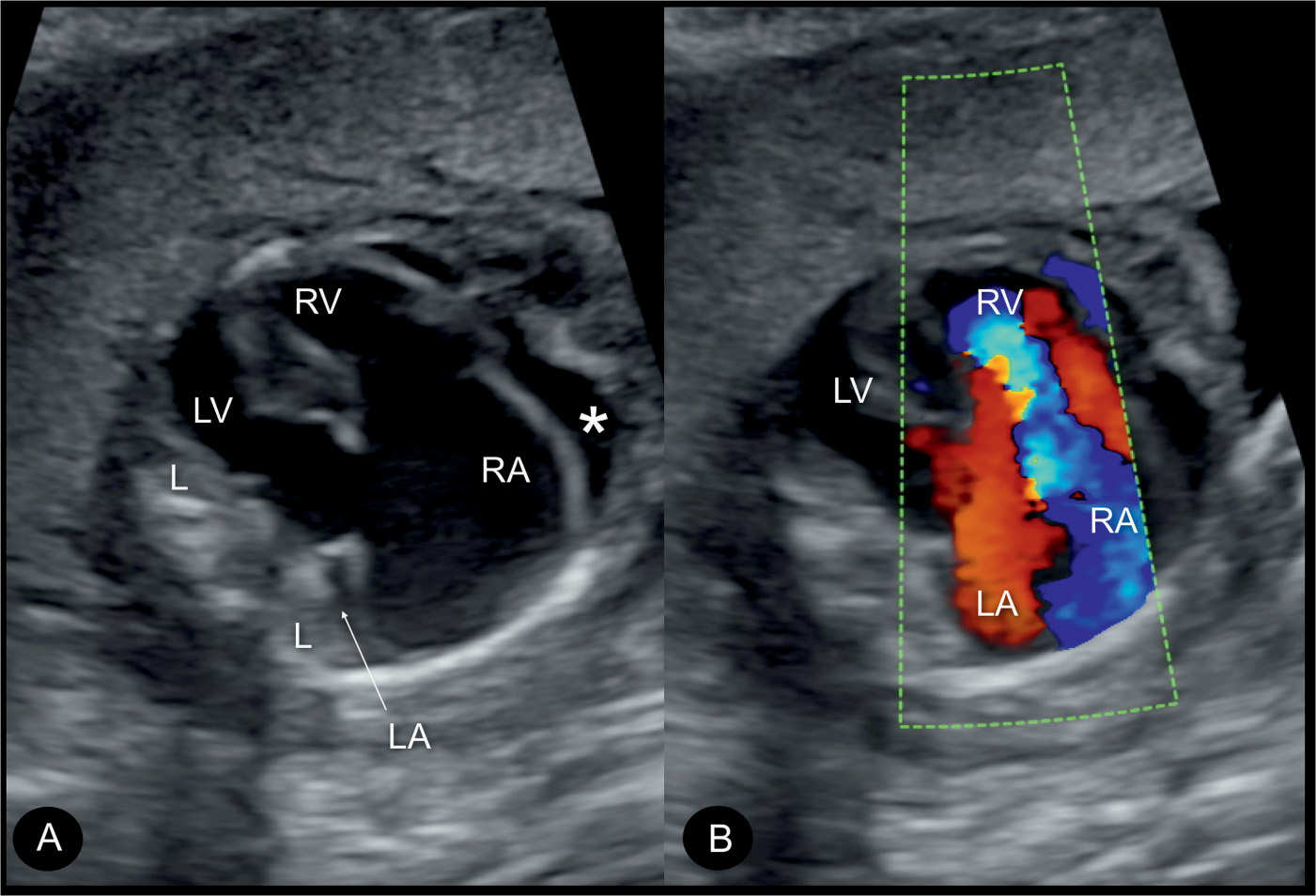
Figure 20.4: Apical four-chamber views in gray scale (A) and color Doppler (B) in a fetus with severe Ebstein anomaly with marked cardiomegaly (A and B) and severe tricuspid insufficiency (B). Note that the heart fills nearly the whole chest. Lungs (L) are compressed and small. Compare with lungs in Figure 20.3. The fetus also had hydrops at presentation at 22 weeks, here recognizable as pericardial effusion (asterisk in A). LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle.
Ultrasound Findings
Gray Scale
In Ebstein anomaly, the four-chamber view on grayscale ultrasound shows an enlarged heart size (cardiomegaly) with an increased cardiothoracic ratio (5). The enlarged right atrium accounts for the enlargement of the heart and for the abnormal cardiothoracic ratio (Figs. 20.2 to 20.4). The dilation of the right atrium, however, can be subtle during the second trimester, and a progressive dilation of the right atrium can be noticed with advancing gestation. The attachment of the septal leaflet of the tricuspid valve to the ventricular wall rather than to the valve annulus can be documented on careful observation of the tricuspid valve anatomy in systole and diastole by using the cineloop technique. This observation is essential for differentiating Ebstein anomaly from tricuspid valve dysplasia (see section Tricuspid Valve Dysplasia). In severe Ebstein anomaly, with a large atrialized ventricle, paradoxical movements of the ventricular septum can be observed, with the apical and basal parts of the interventricular septum showing opposite directional movements. When pulmonary stenosis or atresia is present in association with Ebstein anomaly, the pulmonary artery appears smaller than the ascending aorta (Fig. 20.5) and the pulmonary valve may show poor excursion in the short-axis view. Severe forms of Ebstein anomaly have such a pronounced cardiomegaly that the heart fills more than two-thirds of the thoracic cavity and both lungs are compressed resulting in lung hypoplasia (compare Figs. 20.3 and 20.4). Some cases can also result in cardiac failure and hydrops, which can be an additional reason, besides the cardiomegaly, for pregnancy referral.
Color Doppler
In severe cases of Ebstein anomaly with an enlarged heart, color Doppler helps in the confirmation of severe tricuspid regurgitation (Fig. 20.6). In early stages, however, before the right atrium or the whole heart is enlarged, color Doppler can visualize the typical severe regurgitation and leads to the diagnosis (Fig. 20.7). The tricuspid regurgitation occurs during the entire systole (holosystolic), with peak velocities of greater than 200 cm/s (Fig. 20.8). The systolic regurgitant jet of the tricuspid valve typically arises from the middle of the right ventricle in Ebstein anomaly, in contrast to the regurgitant jet of tricuspid dysplasia or other functional tricuspid regurgitations, which arise at the level of the tricuspid valve annulus, an important differentiating point (Fig. 20.6). Color Doppler of the right outflow tract shows either reverse flow in the ductus arteriosus toward the pulmonary valve or antegrade flow into the typically narrow pulmonary trunk when pulmonary atresia or stenosis is present (6).
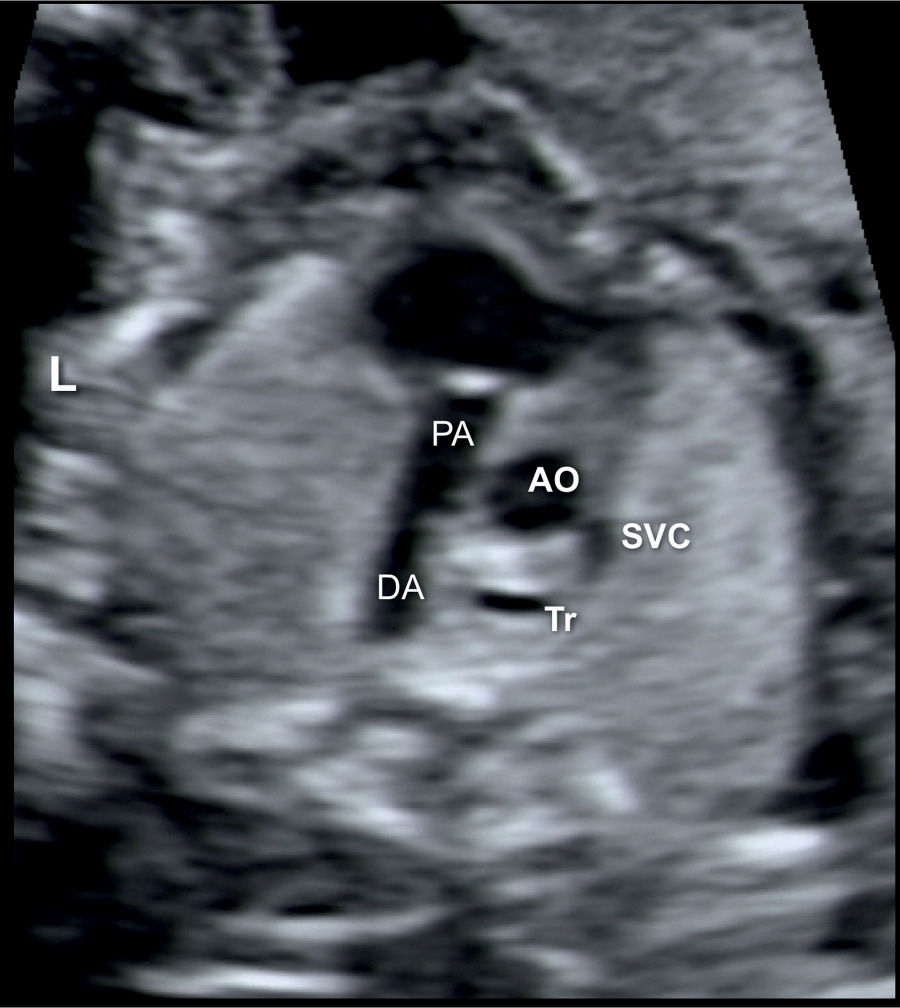
Figure 20.5: Transverse view of the ductal arch (DA) in a fetus with Ebstein anomaly showing right ventricular outflow tract obstruction. Note the narrow pulmonary artery (PA) compared to the size of the ascending aorta (AO). SVC, superior vena cava; Tr, trachea; L, left.
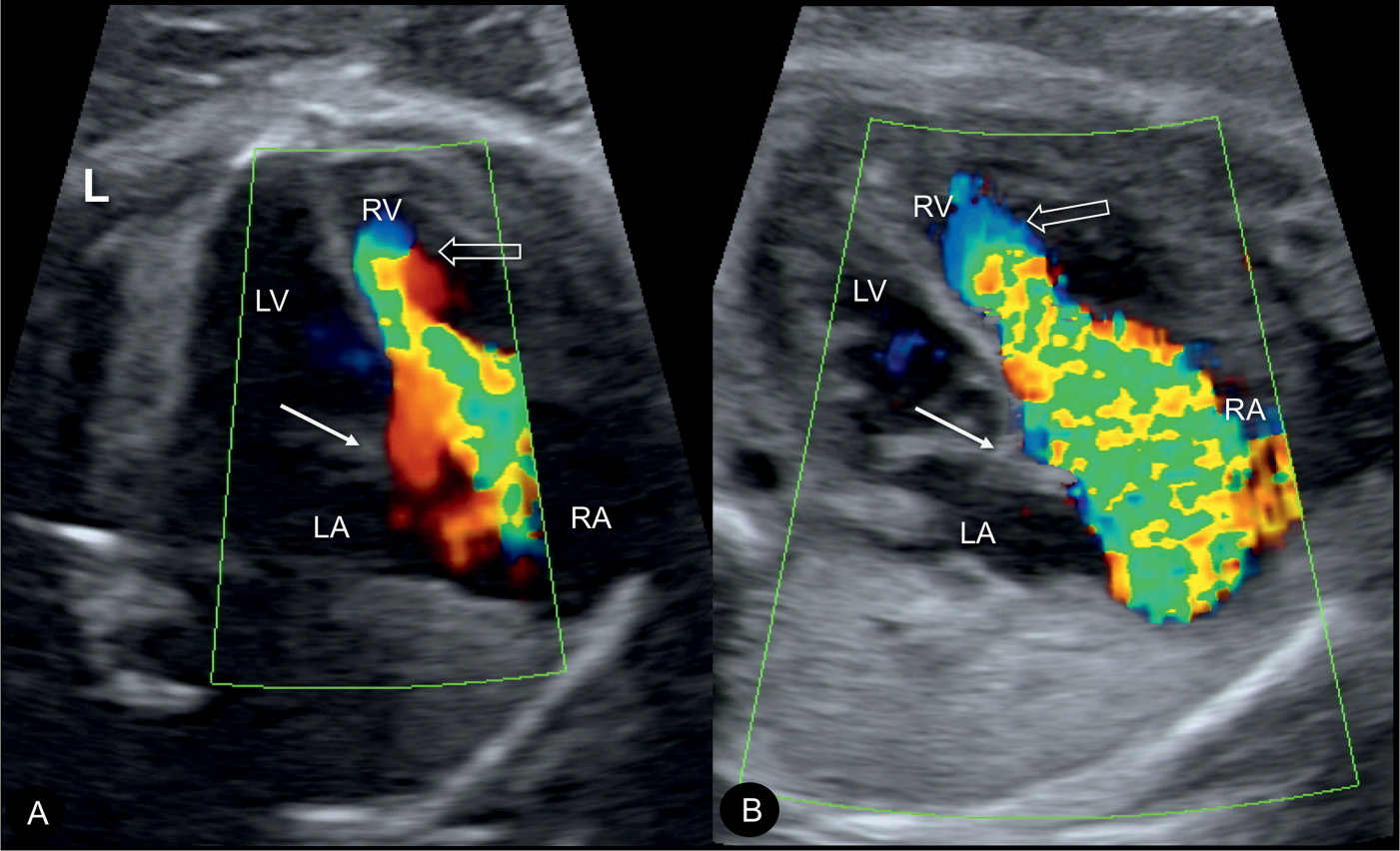
Figure 20.6: Color Doppler during systole at the four-chamber view in two fetuses (A and B) with Ebstein anomaly, demonstrating severe tricuspid regurgitation into the dilated right atrium (RA). Open arrows point to the site of closure of the dysplastic tricuspid valves. Solid arrows point to the attachment of the mitral valves. Note the anatomic origin of the regurgitant jet, deep in the right ventricle (RV), a differentiating feature from tricuspid dysplasia (see text for details). LA, left atrium; LV, left ventricle; L, left.
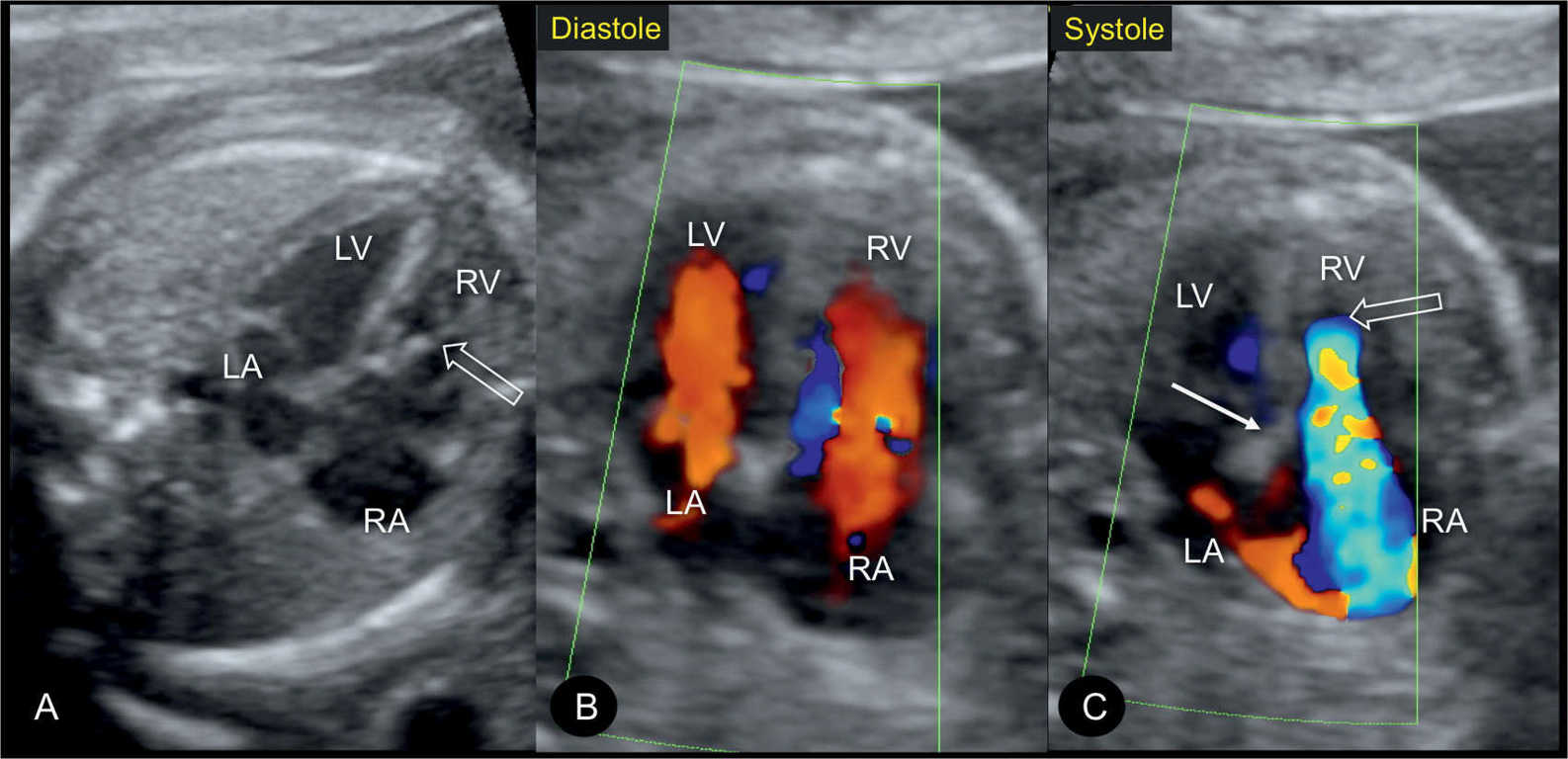
Figure 20.7: Apical four-chamber views in gray scale (A), color Doppler in diastole (B), and color Doppler in systole (C) in a fetus with mild Ebstein anomaly at 22 weeks’ gestation. Note the absence of marked cardiomegaly in A. In diastole (B), color Doppler shows normal atrioventricular filling. In systole (C), severe tricuspid regurgitation is noted with the regurgitant jet starting near the apex of the heart. Open arrows point to the site of closure of the dysplastic tricuspid valves. Solid arrow points to the attachment of the mitral valve. LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle.
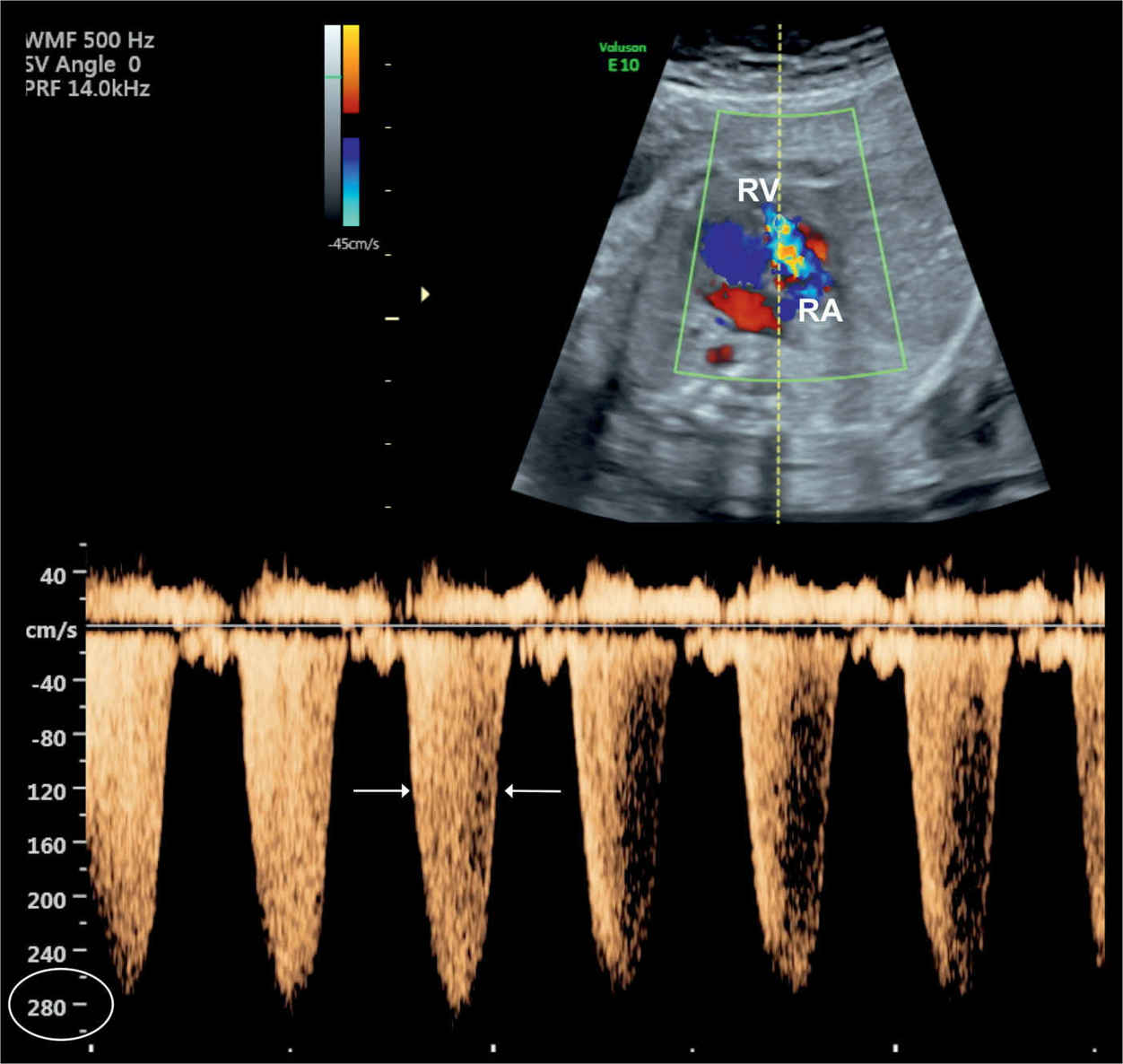
Figure 20.8: Tricuspid regurgitation shown on color and pulsed Doppler in a fetus with Ebstein anomaly. Note the holosystolic duration of the regurgitant jet (arrows) with peak velocities exceeding 270 cm/s. RA, right atrium; RV, right ventricle.
Early Gestation
The presence of tricuspid regurgitation can be present at 11 to 14 weeks’ gestation in fetuses with Ebstein anomaly, but the main findings of cardiomegaly and dilated right atrium are typically seen later in gestation. Early severe cases with cardiomegaly may be associated with a thickened nuchal translucency and fetal hydrops (Fig. 20.9), a sign of impending fetal demise. Given that the majority of severe cases of Ebstein are suspected in early gestation, mild Ebstein cases can be missed in utero and are detected in infancy or even later into adulthood (7).
Three-Dimensional Ultrasound
The display of Ebstein anomaly with three-dimensional (3D) ultrasound, such as tomographic imaging or orthogonal planes, can demonstrate in one view the cardiomegaly, the level of attachment of the tricuspid valve leaflets, and the diminutive pulmonary artery. Surface rendering (Fig. 20.10) can provide a better assessment of the abnormal valve anatomy (8) and could, in the future, be of importance in counseling parents for the options of postnatal therapy. Tricuspid regurgitant jets can be displayed in surface rendering and glass-body mode (Fig. 20.11) (9). Volume measurements of the functional right ventricle may be of help in the future in assessing fetuses at risk for poor prognosis.
Associated Cardiac and Extracardiac Findings
Associated cardiac abnormalities include an obstruction of the right ventricular outflow tract as pulmonary stenosis or atresia (Fig. 20.5) in more than 60% of fetuses diagnosed with Ebstein anomaly prenatally (4). Atrial septal defects represent another common association and have been reported in up to 60% of children with Ebstein anomaly (10). Anecdotal association with congenital corrected transposition of the great arteries or with absent pulmonary valve syndrome were reported as well. The presence of right atrial enlargement and a high prevalence of accessory pathways increase the risk of supraventricular tachyarrhythmia, primarily seen in postnatal studies (11, 12). A large multicenter retrospective study of newborns with Ebstein anomaly, over a 25-year follow-up period, noted a 17% prevalence of rhythm disturbances with supraventricular arrhythmias being the most common (13).
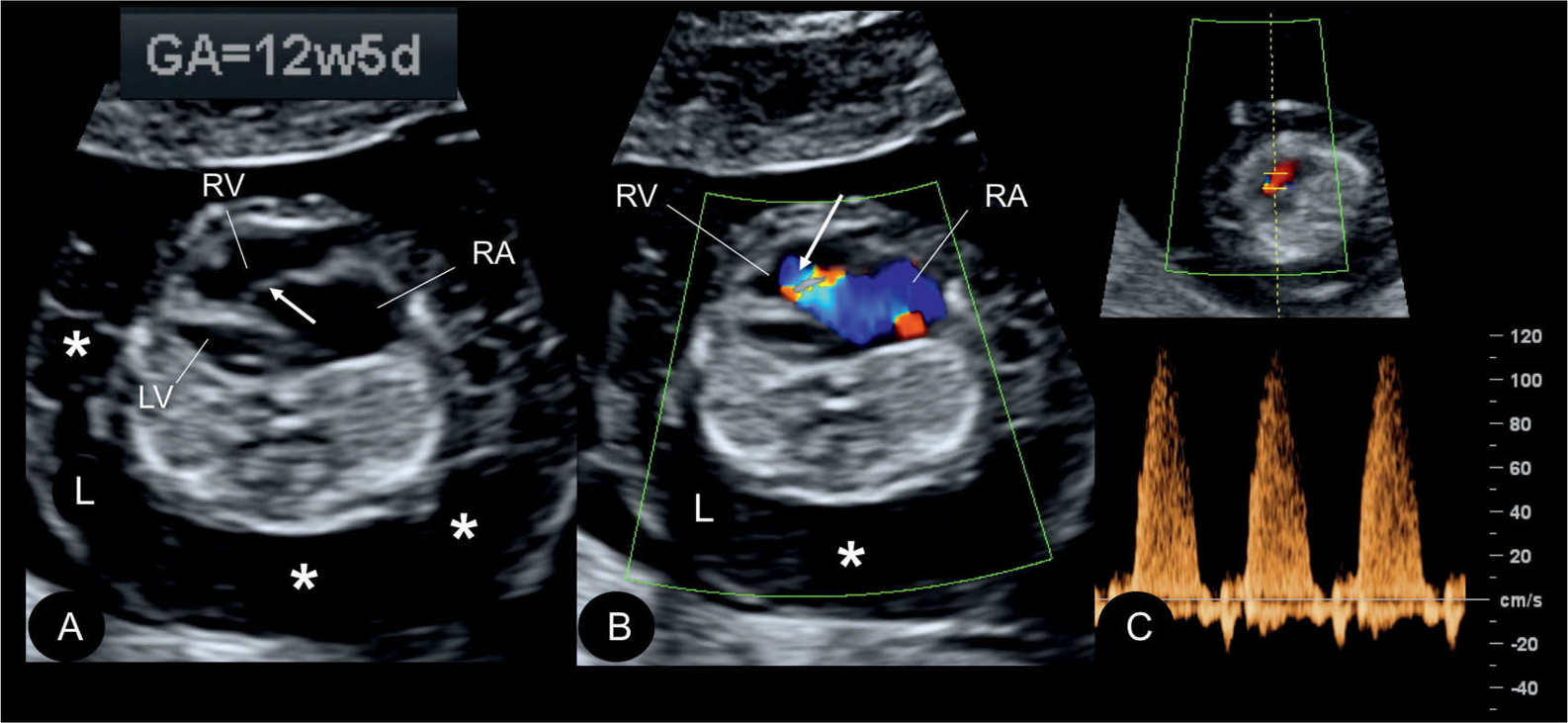
Figure 20.9: Gray scale (A), color (B), and pulsed Doppler (C) in a fetus at 12.5 weeks’ gestation with Ebstein anomaly. Note the presence of generalized hydrops (asterisks) in A and B. The displaced apical attachment of the tricuspid valve in the right ventricle (RV) is shown in A (arrow). Severe tricuspid regurgitation originating near the apex of the RV is shown in B (arrow). Holosystolic tricuspid regurgitation with peak velocities of 120 cm/s is demonstrated in C. RA, right atrium; LV, left ventricle; L, left.
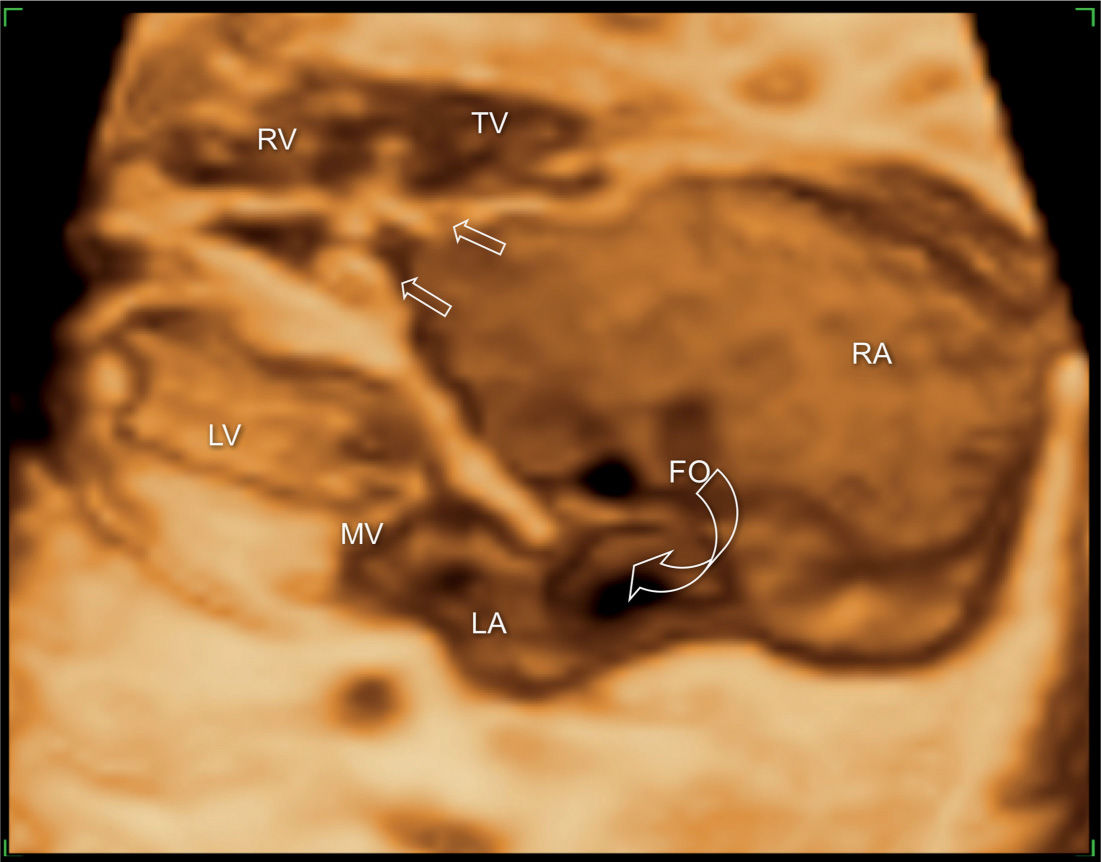
Figure 20.10: 3D ultrasound in surface mode display of the four-chamber view in a fetus with Ebstein anomaly. The large right atrium (RA) and the wide foramen ovale (FO) are demonstrated (open curved arrow). Different levels of attachment of the tricuspid (TV) (open straight arrows) and mitral valves (MV) are noted. LA, left atrium; LV, left ventricle; RV, right ventricle.
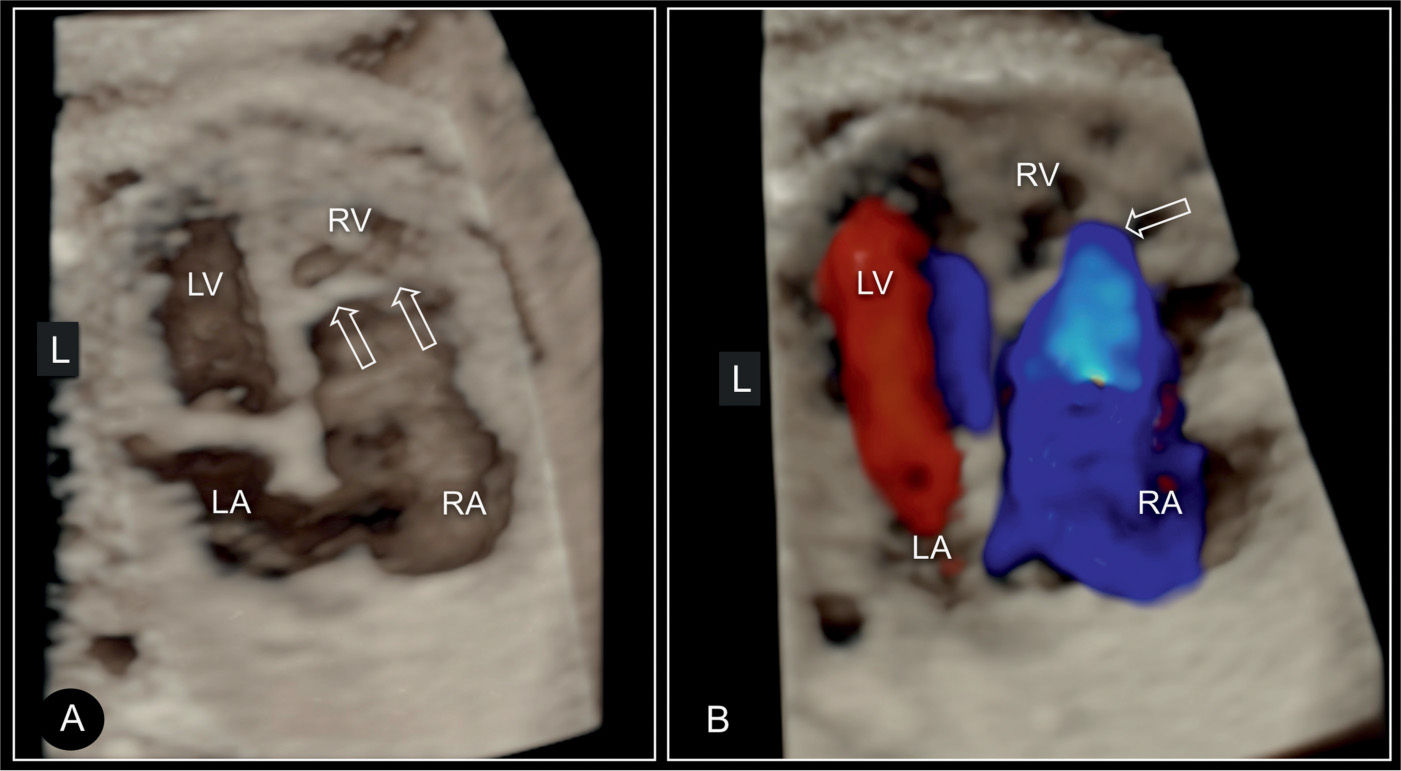
Figure 20.11: 3D ultrasound in surface mode display (A) and glass-body mode (B) of the four-chamber view in a fetus with Ebstein anomaly. Similar to Figure 20.10, the large right atrium (RA) and the small right ventricle (RV) are demonstrated in addition to the displaced attachment of the tricuspid (TV) (open arrows) in comparison to the mitral valves (MV). In B, color Doppler in systole shows the severe regurgitation of the dysplastic tricuspid valve. LA, left atrium; LV, left ventricle.
Most cases of Ebstein anomaly are isolated findings (12), but an association with chromosomal anomalies, such as trisomy 21 or trisomy 13, has been reported in addition to familial cases. Amniocentesis for diagnostic karyotyping (in addition to microarray studies) or screening with maternal blood for cell-free DNA sequencing should be offered as part of the workup. Severe tricuspid regurgitation can lead to in utero cardiac failure and development of fetal hydrops, which may be the first sign of the cardiac abnormality. Pulmonary hypoplasia, which increases neonatal morbidity and mortality, can occur when severe cardiomegaly is found (Fig. 20.4). A cardiothoracic area ratio of greater than 0.6 in fetuses with cardiomegaly is associated with the postnatal presence of pulmonary hypoplasia (5).
Differential Diagnosis
On occasion, it may be difficult to differentiate prenatally between Ebstein anomaly and tricuspid valve dysplasia. The origin of the tricuspid regurgitant jet may help in differentiating these two lesions. In tricuspid valve dysplasia, the jet arises from the normally inserted tricuspid valve at the level of the annulus, whereas in Ebstein anomaly, the origin of the regurgitant jet is displaced inferiorly within the right ventricle owing to the low insertion of the septal and posterior tricuspid valve leaflets (Figs. 20.6 and 20.7). Severe tricuspid regurgitation with cardiomegaly can be found in dilative cardiomyopathy and in other noncardiac lesions with fetal hemodynamic impairment. Premature closure of the ductus arteriosus may also be present with tricuspid regurgitation. Color and pulsed Doppler velocities across the ductus arteriosus help in differentiating this entity from Ebstein anomaly.
Prognosis and Outcome
Several prenatal series of Ebstein anomaly reported poor prognosis, with about 45% of fetuses dying in utero and an overall 80% to 90% mortality (14, 15). Poor prognostic markers prenatally include massive cardiomegaly, decreased right ventricular outflow due to pulmonary stenosis, and fetal hydrops (14, 16) (Fig. 20.9). Compression of the lungs may contribute to pulmonary hypoplasia, a significant risk factor for the neonate. Prenatal diagnosis of Ebstein anomaly is associated with a poor outcome given an inherent selection of the most severe cases. The authors observed that when cardiomegaly is detected in early gestation, especially before 20 weeks, the prognosis is worsened.
An echocardiographic grading score for neonates with Ebstein anomaly was proposed that involves calculating the ratio of the combined area of the right atrium and atrialized right ventricle to that of the functional right ventricle and left heart in a four-chamber view at end diastole (17) (Fig. 20.12). Four grades of increasing severity were described and are shown in Table 20.1 with corresponding outcome (17). Left heart abnormalities involving the myocardium or valves were observed in 39% of primarily adult patients with Ebstein anomaly in one study, suggesting that Ebstein anomaly should not be regarded as a disease confined to the right side of the heart (18). In a review of 37 fetuses with Ebstein anomaly (n = 26) and tricuspid valve dysplasia (n = 11), anterograde flow through the pulmonary valve on the first fetal echocardiography was associated with a good outcome and a retrograde flow was strongly associated with fetal or neonatal death (19). Another study on the perinatal course of Ebstein anomaly and tricuspid valve dysplasia in 21 fetuses (17 with Ebstein and 4 with tricuspid valve dysplasia) revealed a shorter combined isovolemic contraction and relaxation time for nonsurvivors compared with survivors when subanalysis of the left ventricular myocardial performance index was performed (20).
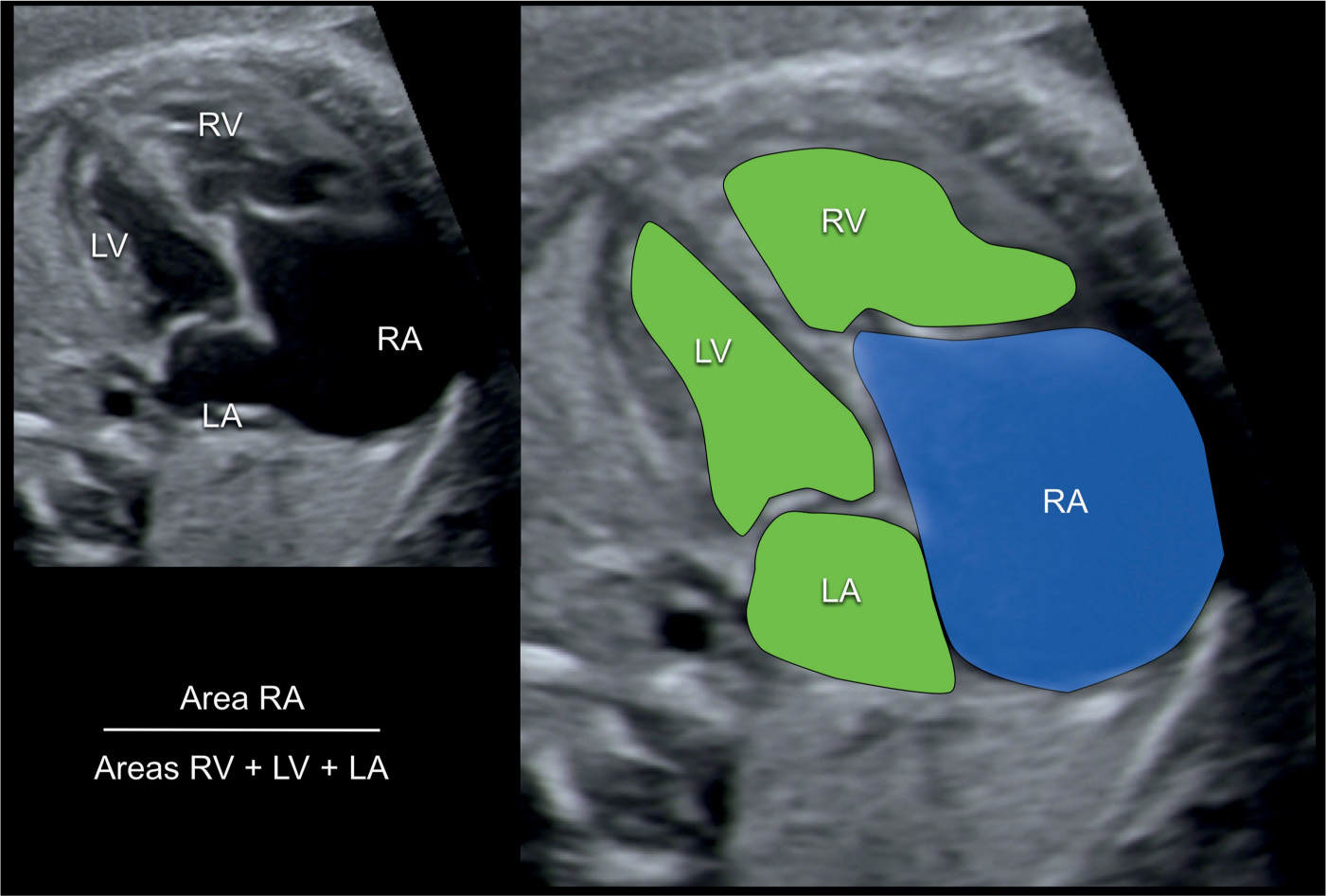
Figure 20.12: Calculation of the prognostic score for outcome of fetuses with Ebstein anomaly. An apical four-chamber view is shown in A and enlarged and colored in B. The area of the right atrium (RA) with the atrialized ventricle is measured (blue in B) and divided by the sum of the area of the remaining right ventricle (RV), the left atrium (LA) and the left ventricle (LV) (green in B). A score below 0.5 predicts a favorable outcome whereas a score greater than 1.5 signalizes very poor outcome. See Table 20.1 for details.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


