Development of Renal Function
Tino D. Piscione
A clear understanding of changes in glomerular and tubular function and renal hemodynamics in the perinatal period is crucial for appropriate management of fluid and electrolyte problems in the sick neonate. In this chapter, general and specific aspects of renal function as they relate to the antenatal and postnatal periods are reviewed to provide a framework for evaluating renal function in the healthy and sick newborn infant.
ANATOMICAL CONSIDERATIONS REGARDING FUNCTIONAL KIDNEY DEVELOPMENT
Morphologic aspects of human kidney development are discussed in detail in Chapter 464. Here, attention is given to spatiotemporal relationships between anatomical and functional development of renal structures. Table 465-1 provides a summary of relationships between anatomical and functional kidney development.
Human kidney development begins at the fifth week of gestation (Fig. 465-1).1,2 The first functioning nephrons are formed by week 9 and excrete urine by week 12. By 32 to 34 weeks, nephrogenesis is completed, following which no new nephron units are formed.3,4 In humans who suffer fetal or perinatal renal injury, the developing kidney is incapable of compensating for irreversible nephron loss by either accelerating the rate of nephron formation ex utero in infants born prematurely, or by de novo generation of nephrons once nephrogenesis is completed.3,5
There is increasing evidence that the number of functioning nephrons formed at 32 to 34 weeks’ gestation may have important implications for short-term and long-term renal function. This concept is supported by the association of renal failure in humans with oligomeganephronia, a severe form of renal hypoplasia characterized by small kidneys and disproportionately reduced nephron number.6,7 Additional support is given by the demonstration of reduced glomerular number in humans with primary hypertension and chronic kidney disease.8,9 Quantitative analyses of glomerular number in humans and rodents using stereological methods of glomerular counting in renal autopsy or necropsy specimens have revealed a direct relationship between birth weight and glomerular number.10,11 The latter data are consistent with the Barker hypothesis, which proposes that adult disease has fetal origins and is based on epidemiological studies showing a correlation between birth weight and the incidence of cardiovascular disease.12,13
Table 465-1. Relationships Between Anatomical Kidney Development and Functional Kidney Development
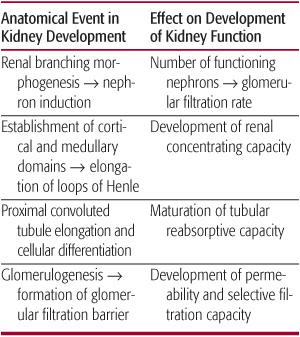
FIGURE 465-1. Schematic representation of the relationship between nephron formation and gestational age during human fetal renal development. Renal branching morphogenesis, a principal determinant of nephron number (solid line), is complete by midgestation. Renal mass (dashed line) increases exponentially in the latter half of gestation through the additional induction of new nephrons and hypertrophy of existing nephrons. Blue shading represents postnatal period.
Between the 22nd and 34th weeks of human fetal gestation,3 or gestation day 15.5 to postnatal day 7 in mice,14 the peripheral (ie, cortical) and central (ie, medullary) domains of the developing kidney are established. The specification of cortical and medullary domains is essential to eventual function of the mature collecting duct system. During embryonic life, the developing renal cortex and medulla exhibit distinct axes of growth. The renal cortex grows along a circumferential axis, resulting in a 10-fold increase in its volume while preserving relative spatial organization of developing structures within the expanding renal cortex.22 In this manner, differentiating glomeruli and tubules maintain their relative position in the renal cortex with respect to the external surface of the kidney, or renal capsule, throughout development and into postnatal life. The preservation of this spatial relationship between developing nephrons and the renal capsule appears to be crucial, as revealed by defective nephron development in mice that fail to form a renal capsule.23
In contrast to the circumferential pattern of growth exhibited by the developing renal cortex, the developing renal medulla expands 4.5-fold in thickness along a longitudinal axis perpendicular to the axis of cortical growth.22 This pattern of renal medulla growth is largely due to elongation of outer medullary collecting ducts.22 Longitudinal growth of the medulla contributes to lengthening of the loops of Henle such that all but a small percentage of the loops of Henle extend below the corticomedullary junction in full-term newborn infants.3 As the kidney increases in size postnatally, the loops of Henle further elongate and reach the inner two thirds of the renal medulla in the mature kidney. In the mature kidney, the loops of Henle contribute to the kidney’s urine-concentrating mechanism by generating an interstitial medullary tonicity gradient as a consequence of sodium and urea transport along its thick ascending limb (TAL) into surrounding interstitial tissue.  In the extremely premature fetus, the loops of Henle are short owing to the relative distance between the renal capsule and the renal papilla. Consequently, the urine concentrating capacity of the premature kidney is limited by generation of a shallow medullary tonicity gradient.
In the extremely premature fetus, the loops of Henle are short owing to the relative distance between the renal capsule and the renal papilla. Consequently, the urine concentrating capacity of the premature kidney is limited by generation of a shallow medullary tonicity gradient.
All parts of the developing nephron increase in size as they mature. However, the most striking changes exist in the proximal convoluted tubule, which shows increased tortuosity with maturation, and in the loop of Henle, which undergoes elongation.3 The human kidney at birth shows marked heterogeneity in proximal tubule length from the outer cortex to the inner cortex.25 Uniformity in proximal tubule length is achieved by 1 month of life, and the proximal tube continues to lengthen at a uniform rate into early childhood. Increasing proximal tubule length during kidney development correlates strongly with increased absolute sodium reabsorption.26
Glomerulogenesis, which describes formation of the glomerular capillary tuft and differentiation of podocytes, mesangial cells, and glomerular capillary endothelial cells,27 is completed in humans by 32 to 34 weeks’ gestation with cessation of nephrogenesis. Subsequently, factors affecting maturation of the glomerular filtration barrier influence glomerular function in the newborn term and preterm infant. The glomerular filtration barrier is a physiologic module composed of fenestrated glomerular capillary endothelial cells, slit diaphragms that form between adjacent podocyte foot processes, and the glomerular basement membrane, which lies interposed between podocytes and glomerular capillary endothelium (reviewed in Kreidberg27 and Pavenstadt, Kriz, and Kretzler28). Glomerular diameter increases by 40% between birth and 18 years of age.25 These factors are likely to contribute to a maturational increase in glomerular filtration rate that occurs after birth.
DEVELOPMENT OF RENAL BLOOD FLOW AND GLOMERULAR FILTRATION RATE
 DEVELOPMENT OF RENAL BLOOD FLOW
DEVELOPMENT OF RENAL BLOOD FLOW
The kidney receives approximately 2.5% to 7% of combined ventricular output during the last trimester of gestation, whereas the newborn kidney subsequently receives 15% to 18% of total cardiac output after birth.33,34 The mechanism responsible for the age-related increase in renal blood flow at birth is largely attributed to a postnatal drop in renal vascular resistance.37
Factors affecting the drop in renal vascular resistance include changes in intrarenal blood flow distribution during the transition from fetal to postnatal life. Studies of intrarenal blood flow distribution have shown that 100% of juxtamedullary glomeruli situated in the inner regions of the renal cortex receive blood flow, whereas less than 25% of glomeruli located within the outer renal cortex are perfused.30 At birth, however, the proportion of perfused outer glomeruli abruptly increases to 77% and reaches 98% within 3 days.
Increased neuroadrenergic system activity likely contributes to increased fetal and neonatal renal vasomotor tone. Immediately following birth in sheep and swine, renal sympathetic nerve activity is high and plasma epinephrine and norepinephrine levels rise severalfold.40,41
The renin-angiotensin system appears to play an important role in regulating fetal renal blood flow. Plasma renin, angiotensin II, and angiotensin converting enzyme (ACE) levels are higher in the first 2 weeks of life than in adulthood.46-48 Expression studies of angiotensin receptors in fetal and newborn sheep kidneys reveal that angiotensin I receptors are upregulated during fetal maturation and are increased 2-fold above adult levels at 2 weeks of age in rats.47 Maturational changes in angiotensin receptor expression may explain the enhanced renal vasoconstrictor response to angiotensin II in newborn fetal sheep as compared to fetal sheep.49
In the early postnatal period, levels of endothelin-1 (Et1), a potent vasoconstrictor, are high in term and preterm newborns.50 Similarly, increased levels of high-affinity Et1-binding sites have been described in 1-day-old rat kidney.51 Lower levels are detected at 30 days of postnatal life,51 suggesting that the vasoconstrictor effect of Et1 on renal blood flow becomes attenuated with advancing postnatal age. 
Prostaglandins, which exert vasodilatory effects on renal blood flow,54 may be involved in counterbalancing vasoconstrictor effects of neuroadrenergic, angiotensin, or Et1-A receptor activation in the kidney during the transition from fetal to postnatal life. Newborns are shown to exhibit elevated circulating levels of prostaglandins and demonstrate high synthetic activity of renal prostaglandins with advancing gestational age.55 The role of prostaglandins in maintaining renal blood flow is suggested by the demonstration of a transient reduction in renal blood flow following administration of the prostaglandin synthetase inhibitor indomethacin to fetal and newborn animals.56,57
The kallikrein-kinin system generates brady-kinin, a potent local vasodilator, through proteolytic cleavage of high molecular weight kininogen.60 Renal expression of kallikrein rises rapidly in the immediate postnatal period.61,62 The possibility that bradykinin promotes renal blood flow in the newborn kidney is suggested by the demonstration in newborn rabbits that bradykinin-2 receptor blockade induces renal vasoconstriction.63
 DEVELOPMENT OF RENAL BLOOD FLOW AND GLOMERULAR FILTRATION RATE
DEVELOPMENT OF RENAL BLOOD FLOW AND GLOMERULAR FILTRATION RATE
Glomerular filtration refers to the formation of a plasma transudate across the glomerular filtration barrier, which ultimately produces urine. Glomerular filtration rate (GFR), the volume of plasma filtered by the kidney per unit of time, provides a measure of the kidney’s filtrative capacity. In clinical practice, GFR is considered the sum total GFR of all functioning nephrons in the kidney (individually referred to as single nephron GFR, or SNGFR).
At birth, GFR, whether expressed per unit of body weight or surface area, is low in mammalian species and correlates closely with gestational age.38,66 In humans, GFR as determined by creatinine clearance is approximately 4 to 8 mL/min/1.73 m2 in newborns of 28 weeks’ gestation and 12 to 40 mL/min/1.73 m2 in newborns born at 40 weeks’ gestation (Fig. 465-2).67,68 Immediately following birth, GFR as determined by creatinine clearance increases 2-fold to 3-fold in term infants in the first months of life.66,69 The sharp postnatal rise in GFR is largely attributed to increased mean arterial blood pressure, increased renal blood flow, and redistribution of intrarenal blood flow32,35,70 (eTable 465.1  ).
).
Single nephron GFR (SNGFR) is influenced by hydrostatic and oncotic pressure gradients across the glomerular filtration barrier. In the adult, changes in glomerular hydrostatic pressure rarely play a significant role in alteration of SNGFR because autoregulatory mechanisms sustain intraglomerular hydrostatic pressure during periods of mild to moderate systemic hypotension.73 In contrast, SNGFR in the neonate is more sensitive to changes in systemic blood pressure than in the adult, since newborn rats exhibit systemic blood pressure that is below the threshold for autoregulation.71,72 Similarly, SNGFR in newborn infants is likely to be affected by lower plasma protein concentrations as measured in neonates, which ultimately results in a reduced oncotic pressure gradient across the glomerular filtration barrier. 
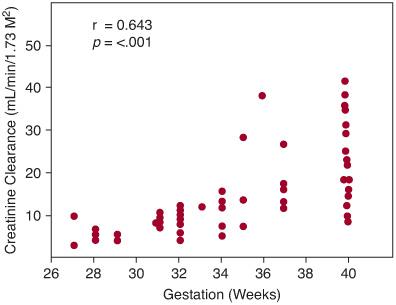
FIGURE 465-2. Creatinine clearance at 24 to 40 hours after birth in neonates of 27 to 40 weeks’ gestation.
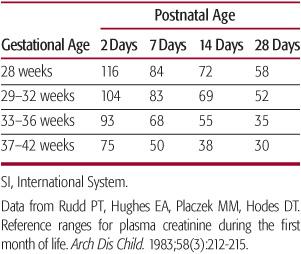
Within the first 48 hours of life, plasma creati-nine concentration reflects maternal rather than newborn concentrations.68 By 7 days of age, plasma creatinine in term neonates is normally less than 45 µmol/L (0.5 mg/dL).78 In preterm neonates, levels can be, on average, as high as 50 to 60 µmol/L throughout the first month of life78 (Table 465-2). A progressive rise in serum creati-nine over time in the neonate suggests a reduction in GFR regardless of gestational age.
WATER HOMEOSTASIS IN THE NEWBORN 
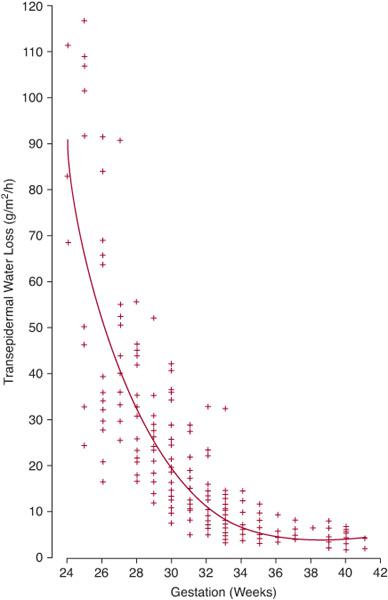
Water conservation is one of the most important functions of the mature mammalian kidney.79 For the developing fetus, regulation of water balance is crucial because total body water may account for up to 70% to 90% of total body mass.79 During gestation, the placenta, not the fetal kidney, serves as the principal regulator of fetal fluid homeostasis.80 Similar to the adult scenario, water homeostasis in the term and premature newborn is determined by factors affecting water intake and output (Table 465-3). Figure 465-3 illustrates the inverse relationship between gestational age and transdermal water loss,87 signifying that the principle determinant of water balance in the first days of life for extremely premature infants (ie, ≤ 30 weeks) is the magnitude of insensible fluid losses.
Similar to the adult scenario, water homeostasis in the term and premature newborn is determined by factors affecting water intake and output (Table 465-3). Figure 465-3 illustrates the inverse relationship between gestational age and transdermal water loss,87 signifying that the principle determinant of water balance in the first days of life for extremely premature infants (ie, ≤ 30 weeks) is the magnitude of insensible fluid losses.
The first days of life are characterized by a physiological state of negative water balance (see Table 465-4).79,88 Water loss is reflected by a 1% to 2% loss in total body weight per day over the first 5 days of life in newborn term infants89 and is greater and more sustained in premature infants.90 Volume contraction during this phase is isotonic and originates from the extracellular fluid compartment without compromising plasma volume.79,91 Atrial natriuretic peptide has been implicated in the immediate postnatal diuresis observed in term as well as preterm infants, although the precise mechanisms of isotonic volume contraction during this period are not clearly understood.92
Table 465-2. Mean Plasma Creatinine Values (SI Units—[µmol/L]) in the Perinatal Period for Different Gestational Ages
The concentrating capacity of the newborn kidney is limited. In the first weeks of life, maximum urine concentrations achieved by preterm and term infants following fluid restriction are 600 mOsm/kg and 800 mOsm/kg respectively94; adult levels (1200 mOsm/kg) are not reached until 6 to 12 months of age95 (eFig. 465.3  ). The ability of the newborn kidney to concentrate urine is highly dependent on development of the loops of Henle.
). The ability of the newborn kidney to concentrate urine is highly dependent on development of the loops of Henle.  By exploiting differential permeabilities to water and solute in its descending and ascending limbs, respectively, the loop of Henle of the fully developed kidney transports sodium and urea out of its thick ascending limb (TAL) and into the surrounding interstitium, which generates an interstitial medullary tonicity gradient for favorable reabsorption of urinary water in the collecting duct.96 The relationship between the length of the loops of Henle and the magnitude of the interstitial tonicity gradient is such that long loops generate a steeper gradient.97,98 Consequently, it is generally held that maturation of urine concentrating ability is functionally coupled to elongation of the loops of Henle that occurs during postnatal kidney growth. In addition, lower rates of sodium and urea delivery and uptake have been described in the TAL of newborn infants68,99-102 (eTable 465.2
By exploiting differential permeabilities to water and solute in its descending and ascending limbs, respectively, the loop of Henle of the fully developed kidney transports sodium and urea out of its thick ascending limb (TAL) and into the surrounding interstitium, which generates an interstitial medullary tonicity gradient for favorable reabsorption of urinary water in the collecting duct.96 The relationship between the length of the loops of Henle and the magnitude of the interstitial tonicity gradient is such that long loops generate a steeper gradient.97,98 Consequently, it is generally held that maturation of urine concentrating ability is functionally coupled to elongation of the loops of Henle that occurs during postnatal kidney growth. In addition, lower rates of sodium and urea delivery and uptake have been described in the TAL of newborn infants68,99-102 (eTable 465.2  ).
).
In addition, several studies have suggested that reduced urine-concentrating capacity in the newborn may be due to relative resistance of the immature kidney to antidiuretic hormone (ADH, or arginine vasopressin).109,110 The mechanism for ADH resistance is believed to be caused by local prostaglandin production, which exerts a direct antagonistic effect on ADH signaling in the neonatal collecting duct.
DEVELOPMENT OF RENAL SODIUM HOMEOSTASIS
In the first week of life, fractional excretion of sodium, expressed as the percentage of filtered sodium excreted in the urine, is high and inversely proportional to gestational age (eFig. 465.4  ).115 This natriuretic state provides for the isotonic extracellular volume contraction observed in newborns and is considered physiologic. A reduction in fractional excretion of sodium subsequently occurs by the second and third weeks of life, contributing to a positive sodium balance that is essential for growth.116,117
).115 This natriuretic state provides for the isotonic extracellular volume contraction observed in newborns and is considered physiologic. A reduction in fractional excretion of sodium subsequently occurs by the second and third weeks of life, contributing to a positive sodium balance that is essential for growth.116,117
During fetal and postnatal kidney development, there is progressive maturation of each nephron segment involved in sodium transport, which include the proximal convoluted tubule, the descending and ascending limbs of the loop of Henle, and the distal convoluted tubule118,119 (eFig. 465.5  ).
).
Table 465-3. Factors Affecting Water Balance in the Sick and Healthy Neonate

Proximal tubule sodium reabsorption in the mature kidney occurs predominantly via sodium-dependent organic solute transporters that reclaim filtered glucose, amino acids, or phosphate. Basolateral Na+,K+-ATPase activity provides a gradient for sodium ion (Na+) entry into the proximal tubule epithelial cell by these transport mechanisms. The reabsorption of positively charged sodium ions in this context generates a lumen-negative transepithelial potential difference, which provides a driving force for passive paracellular chloride transport. In the late proximal tubule, sodium chloride (NaCl) transport is coupled to hydrogen ion (H+) secretion by operation of the Na+/H+ exchanger (NHE3).
In the newborn kidney, sodium reabsorption is mitigated by decreased expression levels of all solute transporters, Na+,K+-ATPase, and NHE3.120-123 In addition, passive paracellular reabsorption of sodium chloride is significantly impaired in the newborn kidney because the neonatal proximal tubule is impermeable to chloride.124 Age-related differences in tubular reabsorptive capacity seem to account for higher urinary concentrations of glucose and amino acids in premature and term infants.126,127 Glucocorticoids appear to play an important role in promoting the drop in fractional sodium excretion that occurs after birth by inducing Na+,K+ATPase and NHE3 expression in the developing proximal tubule.120,128-130 Thyroid hormone may also play a role in tubular maturation.
Active transcellular sodium uptake in the mature loop of Henle occurs via the luminal Na+K+-2Cl− cotransporter (NKCC2) and NHE3 in the thick ascending limb (TAL). Na+ is also absorbed via the paracellular pathway due to the lumen-positive voltage caused by recycling of potassium ions (K+) via renal outer medullary potassium (ROMK) channels into the TAL lumen.135
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


