Delivery Room Resuscitation of the Newborn
BACKGROUND
Resuscitation is derived from the Latin word resuscitare, meaning “to revive.”1 Although the majority of births involve no or little intervention on behalf of the neonate, approximately 10% of newborns need some form of resuscitation, and 1% require extensive maneuvers, such as endotracheal intubation, intravascular access, and drug delivery in the delivery room (DR).2 The transition from fetus to neonate requires a number of physiologic changes, most of which must happen immediately in the seconds and minutes after birth. When these transitions fail to occur, or the fetus has been compromised because of intrinsic disease or utero events, resuscitation is necessary to optimize the chances of a normal outcome.
This chapter focuses on resuscitation of the neonate, not of the fetus or of the mother. Similarly, it does not cover topics more appropriate to stabilization, such as management of glucose homeostasis and electrolyte disorders. Although limited in scope to neonatal resuscitation within the context of the first minutes of life immediately after birth, many of the concepts covered in the chapter are also applicable to neonatal resuscitation in other environments and at later points in the life of the infant. This chapter does not attempt to replicate the definitive resource on neonatal resuscitation, the Textbook of Neonatal Resuscitation published by the American Academy of Pediatrics (AAP) and the American Heart Association (AHA) as the reference work for the Neonatal Resuscitation Program (NRP).3 Rather, seeks to provide an overview of neonatal resuscitation, discussing not only the current state of the scientific evidence and clinical guidelines but also the gaps in present knowledge and the developments likely to occur in the future.
DEVELOPMENT OF NEONATAL RESUSCITATION GUIDELINES
The management of neonates in the DR may have far-reaching consequences, not only on their subsequent neonatal course but also on their long-term outcome. Ideally, all of the interventions undertaken in the DR should be based on the best-available scientific evidence. However, prospective, sufficiently powered, randomized controlled trials (RCTs) of interventions undertaken during neonatal resuscitation are uncommon. Neonatal resuscitation involving more than drying, warming, stimulation, suctioning, and brief positive-pressure ventilation (PPV) is a low-frequency event; in addition, it is hard to anticipate, making RCTs with or without informed consent difficult to undertake. So, how are guidelines for neonatal resuscitation developed?
The development of neonatal resuscitation guidelines involves 2 distinct processes, each carried out on a quinquennial basis. To determine the best-available evidence on which to base recommendations for clinical care, a review of the published science is undertaken by members of the neonatal delegations to the International Liaison Committee on Resuscitation (ILCOR). ILCOR is an international body of health care professionals (HCPs) from resuscitation councils in the United States, Canada, South America, Europe, Australia, New Zealand, South Africa, and other countries and areas of the world whose members volunteer literally thousands of hours in pursuit of thorough, objective review of the scientific literature on resuscitation.4,5 This international activity results in consensus regarding what the current science reveals on a particular topic pertinent to resuscitation.6,7 To achieve accuracy and objectivity in interpretation of the literature as well as uniformity in the process itself, a number of strategies are employed. Only published manuscripts are reviewed, and only primary data are reported; although review articles may occasionally be examined, the original manuscripts are always reviewed and referenced. The results of each manuscript are rated concerning the level of the evidence (eg, RCTs are rated more highly than retrospective case series) and the quality of the methodology. Strict procedures are in place to encourage transparency and minimize any potential for reviewer bias.
Whereas the goal of the review of the published science is international agreement about what the science says about a particular topic, the process of generation of clinical guidelines is a regional or national activity focused on the pragmatic application of the science to patient care based on available local resources. Because the resources available to HCPs in a developed country exceed those present in the developing world, clinical guidelines may differ substantially between regions of the world. For example, the availability of oxygen raises the issue of how much should be used during various stages of resuscitation; in locations where oxygen is not available, there is no debate, and room air (21% oxygen) is used. In the United States, clinical guidelines are the responsibility of the members of and liaisons and consultants to the NRP Steering Committee (NRPSC).8 Whereas established, accepted practices will remain in the guidelines unless evidence of possible harm or proven ineffectiveness exists, the only new interventions that are added are those that are supported by the evidence review process. In generating clinical guidelines, the NRPSC must consider what is practical not only in levels III–IV neonatal intensive care units (NICUs) but also in any facility in which newborns are delivered. Because every guideline carries potential legal, financial, and ethical implications, the NRPSC carefully debates and scrutinizes each one. These guidelines serve as the de facto national standard of care for newborns in US DRs.
Although the ILCOR process for review of the science readily enables achieving international consensus and generation of clinical guidelines by the NRPSC ensures that such guidelines carefully reflect what is both evidence-based and practical, it nevertheless is far from a nimble activity capable of rapidly incorporating new evidence as it becomes available. The veracity of the evidence evaluation could be enhanced by inviting the original authors of pertinent manuscripts to discuss, pool, and reinterpret their original data, similar to how such reviews are carried out in other domains within health care. New communication technologies and social media may allow for more timely interaction and updating of clinical guidelines (perhaps via an online secure peer-reviewed wiki) while maintaining the meticulous character of the current scientific review process. By constantly reexamining how to enhance this productive but at times tedious and time-consuming process, the care of neonates in the DR will be steadily improved.
IMPLEMENTATION OF NEONATAL RESUSCITATION GUIDELINES
NRP Algorithm Overview
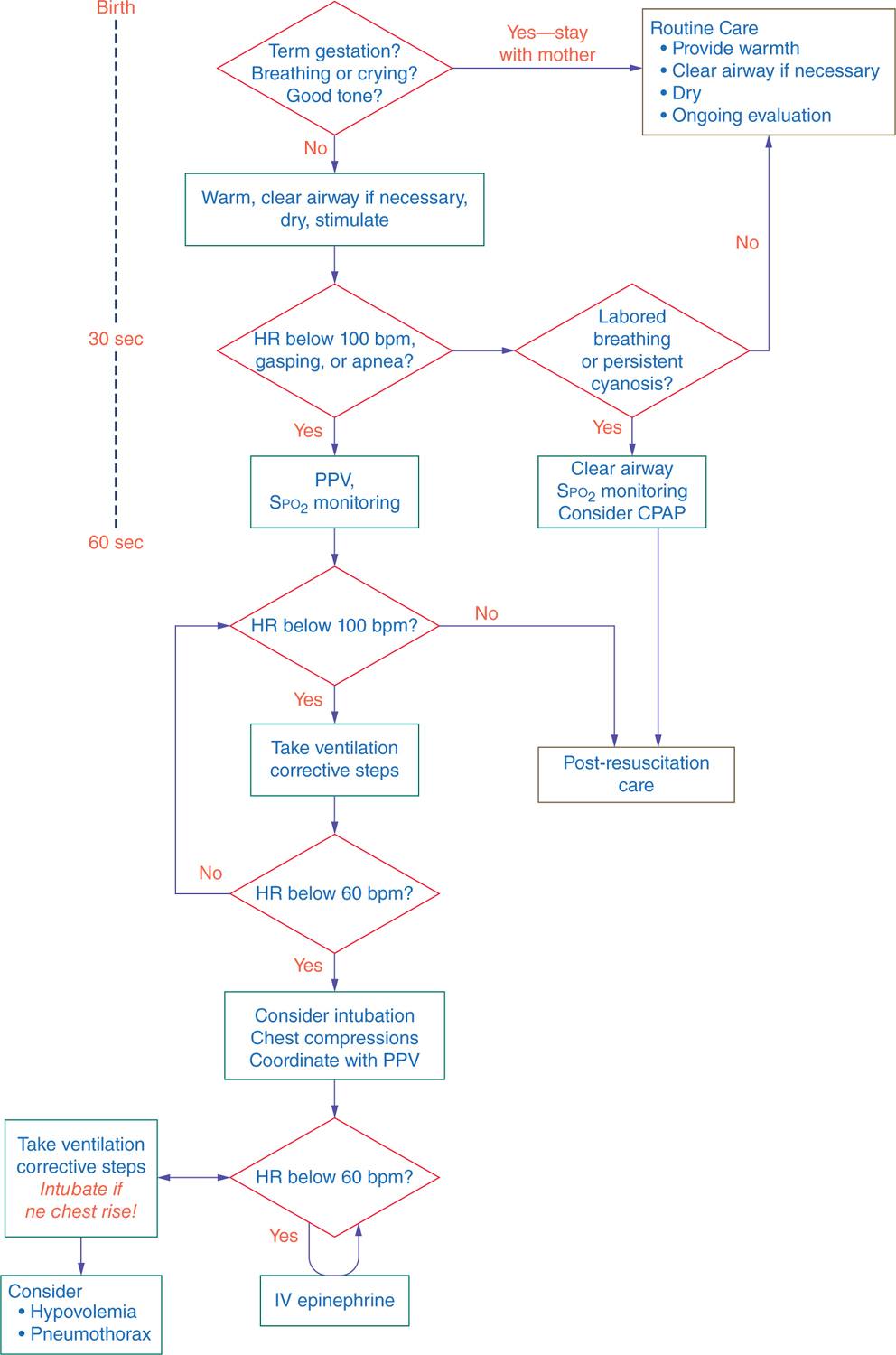
The first steps in resuscitation consist of drying, warming, tactile stimulation, and proper positioning to allow opening of the airway (see Figure 8-1). Neonates should be positioned with the neck slightly extended; this “sniffing” position aligns the posterior pharynx, larynx, and trachea so that air may pass easily through those structures. If, after proper positioning, obstruction of the airway is suspected by the presence of retractions or obvious respiratory effort without evidence of air entry into the lungs, use a bulb syringe or suction catheter to first suction the mouth, then the nose, to remove any large particulate matter or thick secretions. Similarly, suctioning may be undertaken in preparation for PPV. Routine suctioning, however, is not indicated, and neonates who are breathing effectively should simply be dried and warmed.9–12 Vigorous, repetitive, or deep (posterior pharyngeal and gastric) suctioning should be avoided to minimize the risk of vagal stimulation, resultant bradycardia, and direct trauma to the airway. Only when gastric dilation is so significant that it is likely to be impeding diaphragmatic movement and thereby impairing the neonate’s ability to breathe should gastric suctioning be undertaken.
FIGURE 8-1 Neonatal Resuscitation Program (NRP) algorithm for neonatal resuscitation. bpm, beats per minute; CPAP, continuous positive airway pressure; HR, heart rate; PPV, positive-pressure ventilation. (Reproduced with permission from Kattwinkel J, Perlman JM, Aziz K, Colby C, et al. Part 15: neonatal resuscitation: 2010. American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122 (18 Suppl 3):S909–S919.)
Pulmonary Resuscitation
Assisted Breathing
Unlike the pediatric and adult populations, the vast majority of neonates in need of resuscitation do not have an underlying cardiac etiology for their physiologic compromise and thus will respond to interventions that facilitate adequate gas exchange. Perinatal depression may be caused by in utero events, such as inadequate gas exchange at the placental level or postnatal conditions secondary to obstruction of the airways, pulmonary diseases such as surfactant deficiency, and central nervous system disorders causing apnea or hypopnea. Because of this, most neonates who are apneic or manifesting ineffective respirations will respond to the initial steps of the NRP resuscitation algorithm without the need for more invasive measures such as PPV or intubation.
If, after the initial maneuvers described previously are completed, the neonate has labored breathing (eg, substernal and intercostal retractions, grunting), it is reasonable to consider the initiation of continuous positive airway pressure (CPAP). CPAP provides a continuous source of gas flow under pressure as a means of improving alveolar expansion and maintaining functional residual capacity (FRC), thereby decreasing atelectasis, enhancing surfactant release, improving the ventilation/perfusion ratio, and facilitating gas exchange.13 The use of CPAP (together with permissive hypercapnea and avoidance of episodes of hyperventilation) has been shown to result in lower rates of chronic lung disease (CLD), less need for supplemental oxygen, fewer days of mechanical ventilation, and shorter lengths of stay in the preterm population.14–18 This body of work has led to the recommendation by the NRP that CPAP be considered prior to intubation in all spontaneously breathing neonates manifesting respiratory distress. CPAP may be delivered via endotracheal tube (ETT), nasal prongs, or face mask; at the time this chapter was prepared, the optimal method of CPAP delivery as well as range of pressures was yet to be determined.
Neonates who are apneic or hypopneic will not be able to be supported with CPAP and will require PPV delivered via mask, ETT, or laryngeal mask airway (LMA). PPV is initially administered at the rate of 40 to 60 breaths per minute. If the patient begins to breathe in a more effective manner and heart rate (HR) increases, the rate of PPV can be gradually decreased and the patient transitioned to room air, nasal cannula, or CPAP as indicated. Face masks come in different sizes and shapes, and it is important that the mask chosen for a particular neonate cover the patient’s nose and mouth without applying pressure to the eyes; this will result in a proper seal, minimize air leakage, and reduce the risk of vagally induced bradycardia.19–23
An inability to effectively deliver gas into the patient’s lungs should prompt the following steps in the order listed in Table 8-1: The MRSOPA mnemonic is advocated by the NRP to assist HCPs in ensuring that lack of attention to relatively simple issues does not result in unnecessary invasive procedures (such as intubation) and adverse patient outcomes.3
Table 8-1 MRSOPA: Prompts for Technical Interventions When Unable to Deliver Positive-Pressure Breathsa
M: Adjust the mask on the face.
R: Reposition the head to ensure an open airway.
S: Suction the mouth then the nose.
O: Open the mouth and perform a jaw lift.
P: Gradually increase pressure until bilateral breath sounds are auscultated and chest rise is visible, keeping in mind that peak inspiratory pressures (PIPs) above 40 cm H2O are rarely required and likely indicate serious underlying pulmonary pathology.
A: Consider an alternative airway, such as an endotracheal tube (ETT) or laryngeal mask airway (LMA).
aData from Kattwinkel J, Bloom RS, et al: Textbook of Neonatal Resuscitation. 6th ed. Elk Grove Village, IL: American Academy of Pediatrics and the American Heart Association; 2010.
The optimal inspiratory times (TIs) for use during initiation of PPV are unknown. Historically, relatively short initial TIs (0.3–0.5 seconds) have been advocated, but recent studies indicated a benefit of longer (1–3 seconds) TIs on achieving FRC, optimal lung inflation, and adequate HR.24–28 Optimal peak inspiratory pressures (PIPs) vary from patient to patient depending on the underlying pulmonary anatomy and physiology and other variables, such as TI. Because of the fluid-filled nature of the fetal lung and the presence of disease states such as surfactant deficiency, PIPs as high as 30 to 40 cm H2O may need to be delivered initially to establish the FRC. Grunting on auscultation is caused by the neonate exhaling against a partially closed glottis and acts to create end-expiratory pressure to maintain lung inflation. When PPV is delivered, the provision of positive end-expiratory pressure (PEEP) will assist the neonate in establishing FRC and limiting atelectasis.29 The optimal range for PEEP in the neonate is yet to be defined.
When PPV with a mask is unsuccessful, intubation with an ETT is indicated. As with the mask, the ETT must be of an appropriate size for the neonate. Table 8-2 indicates the correlation of ETT size with neonatal weight. The technique of neonatal intubation is illustrated beautifully in color photos in the Textbook of Neonatal Resuscitation3 and in video in the accompanying multimedia DVD. Intubation is a technical skill that is not possessed by all HCPs who attend to newborns in the DR. Because of the difficulty in acquiring and maintaining appropriate skill in intubation, interest in the use of the LMA has grown in recent years.30–34 The advantage of the LMA is in its relative simplicity of insertion and use. The technique of LMA insertion is also illustrated in the Textbook of Neonatal Resuscitation and the accompanying DVD. Currently, there is only one size LMA (size 1) that is available for the neonatal population; it is meant to be used in neonates greater than 2 kg or older than 34 weeks estimated gestational age (EGA). It is especially useful in providing PPV in patients with micrognathia (such as in Pierre Robin sequence) and other craniofacial/airway anomalies that make opening the mouth for placement of a laryngoscope and manipulation of an ETT difficult.
Table 8-2 Recommended Endotracheal Tube Size Based on Patient Weight: Neonatal Populationa

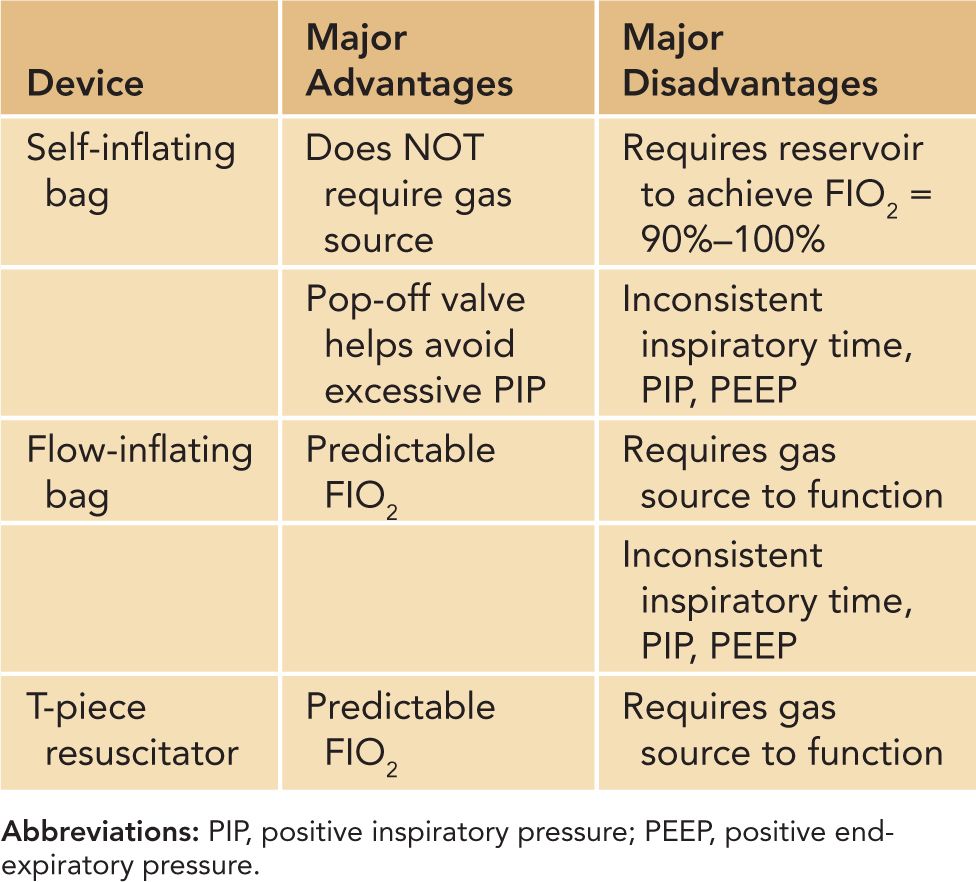
Positive-pressure ventilation (via mask, ETT, or LMA) may be administered using a flow-inflating bag, self-inflating bag, or T-piece resuscitator.35–37 Resuscitation bags have been in use for decades, and each has its own advantages and disadvantages, as noted in Table 8-3. A flow-inflating bag requires a continuous source of compressed gas to function; compression of the bag provides tactile cues that allow the experienced user to sense the compliance of the neonate’s lungs with each breath. Unlike the flow-inflating bag, the self-inflating bag refills spontaneously without the need for a compressed gas source but does not provide the same type of tactile cues. The T-piece resuscitator allows the user to deliver a preset PIP and PEEP and vary the TI and rate of ventilation. A comparison of the flow-inflating bag, self-inflating bag, and T-piece resuscitator in a neonatal mannequin revealed that the T-piece resuscitator delivered a more consistent PIP and together with the flow-inflating bag was found to be more effective at generating a consistent PEEP than the self-inflating bag.38 The subjects in the study described the T-piece resuscitator as requiring the least amount of practice and technical skill to operate in an effective manner.
Table 8-3 Advantages and Disadvantages of Self- and Flow-Inflating Bags and T-Piece Resuscitators
Dosing of Oxygen
Historically, oxygen has been used liberally during resuscitation; until recently, the NRP recommended the use of a 100% fraction of inspired oxygen (FIO2) any time a neonate required respiratory assistance. This dogma began to evolve in the past decade as a number of studies raised questions regarding both the effectiveness of oxygen and its assumed lack of sequelae.39–42 A 2005 meta-analysis by Saugstad et al revealed a more rapid increase in HR, shorter time to first breath, and a 5% reduction in mortality for neonates resuscitated with 21% as opposed to 100% FIO2.43,44 This was followed in 2007 by another meta-analysis, this one by Rabi and colleagues, examining depressed newborns who were resuscitated with 21% vs 100% FIO2; this indicated a lower 1-week and 1-month mortality rate in neonates resuscitated with 21% FIO2.45 The use of oxygen in premature neonates has raised questions that are even more serious because these patients are at higher risk for oxygen toxicity given their diminished ability to mount an adequate antioxidant defense.46,47
Hyperoxia is associated with morbidities like CLD, retinopathy of prematurity (ROP), and necrotizing enterocolitis (NEC). Wang et al performed a prospective, randomized trial of the use of 21% vs 100% FIO2 in preterm neonates at 23–32 weeks gestation and found that all those who were resuscitated with 21% FIO2 failed to achieve predetermined target pulse oximetry (SpO2) levels despite 3 minutes of PPV.48 Escrig et al compared the use of 30% vs 90% FIO2 in neonates less than 28 weeks EGA and determined that a target SpO2 level of 85% at 10 minutes of life could be achieved using 30% FiO2.49 The work of these investigators and others has led the NRP to change its recommendation on the use of oxygen during resuscitation.3 In term neonates, the NRP’s 2010 recommendations are as follows:
1. Begin PPV with 21% FIO2.
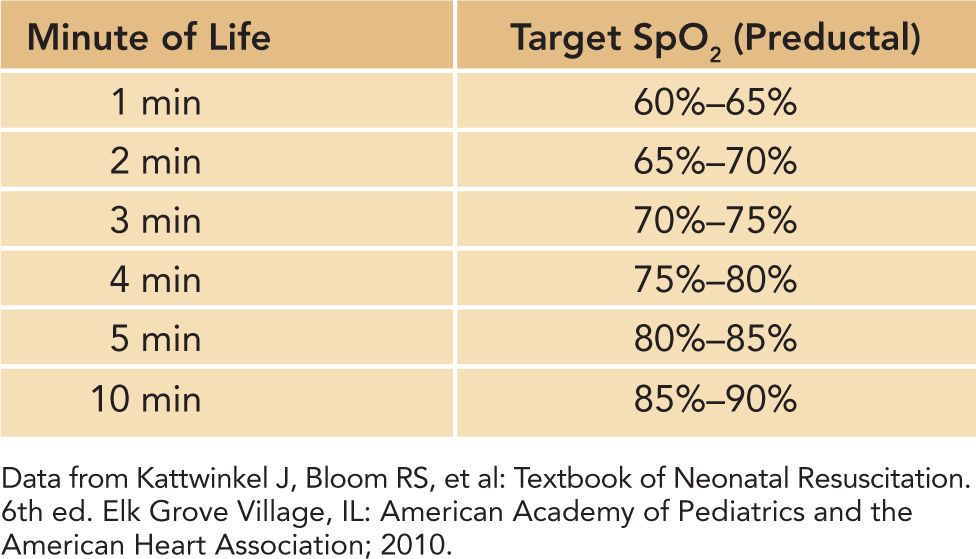
2. Adjust FIO2 to maintain a hemoglobin oxygen saturation by pulse oximetry (SpO2) that mimics that of uncompromised term newborns in the first 10 minutes of life (see Table 8-4).
3. Use a pulse oximeter and oxygen blender to titrate FIO2.
4. Increase FIO2 to 100% whenever chest compressions (CCs) are initiated.
Table 8-4 SpO2 Normal Ranges in the First 10 Minutes of Life
The 2010 NRP guidelines for the use of oxygen in the premature neonate are as follows:
1. When anticipating and planning for a preterm delivery, set up a pulse oximeter and oxygen blender.
2. Begin PPV with an FIO2 somewhat higher than 21% (eg, 30%–40%).
3. Adjust FIO2 to maintain a hemoglobin oxygen saturation by SpO2 that mimics that of uncompromised term newborns in the first 10 minutes of life.
4. Increase FIO2 to 100% whenever CCs are initiated.
Monitoring Oxygen Levels
With the understanding that oxygen should be used in the same manner as any drug and therefore its dose titrated to achieve effect, a means of measuring that effect becomes paramount to delivering appropriate care. Numerous studies have shown that assessment of skin color is an unreliable means of diagnosing cyanosis in the newborn. Detection of abnormal hemoglobin oxygen saturation is best achieved with the use of a pulse oximeter attached via a probe placed on the neonate’s right hand or wrist.50–55 This preductal SpO2 will reflect the hemoglobin oxygen saturation of blood that is perfusing the brain and heart. Pulse oximetry should be used whenever resuscitation is anticipated (as in a preterm delivery); central (lips, tongue, central thorax) cyanosis is persistent; oxygen is in use more than briefly; or PPV is in progress.
Monitoring Carbon Dioxide Levels
The use of end tidal carbon dioxide (ETCO2) detection devices is now recommended by the NRP as a primary means of confirming proper ETT placement. ETCO2 monitoring can be achieved by using colorimetric devices that change color (purple to yellow) in the presence of carbon dioxide (CO2) and capnographs that continuously measure and display CO2 levels via an electrode placed at the connection between the ventilator circuit and the ETT.56–62 Whereas colorimetric devices provide a simple yes/no to the presence of CO2, capnographs will present a continuous measurement of the concentration of exhaled CO2. Despite their utility, it is important for HCPs charged with resuscitating neonates to understand that both devices will give false-negative readings when pulmonary blood flow is low (as in severe bradycardia or asystole). In these situations, other indicators of proper ETT placement, such as auscultation of bilateral breath sounds and visualization of symmetric chest rise, should also be used.
Management of the Meconium-Stained Neonate
Meconium-stained amniotic fluid (MSAF) is encountered in approximately 7%–20% of live births, with 2%–9% of those resulting in meconium aspiration syndrome (MAS). MAS refers to the pulmonary dysfunction in the neonate that may accompany the presence of MSAF in the lungs; it is characterized by impaired oxygenation and ventilation caused by obstruction of the larger airways, with resultant under- and overinflation of the alveoli, a generalized pulmonary inflammatory response, and ventilation-perfusion mismatching, all of which act to limit gas exchange. The relative consistency of the meconium does not influence the rate or severity of MAS. Until recently, the standard approach to the neonate with MSAF consisted of aggressive suctioning of the oro- and nasopharynx while the neonate’s head was on the perineum prior to delivery of the body, followed by intubation and suctioning of the trachea.63–65 Much effort was made to accomplish all of this suctioning prior to the first breath to “prevent” meconium present in the trachea from being aspirated deeper into the tracheobronchial tree and increasing the risk of MAS.
Better imaging of fetal activity indicated that fetal breathing movements occur in utero, effectively moving amniotic fluid in and out the lungs; it also was recognized that fetuses in distress will both pass meconium and manifest gasping in utero, thus creating a mechanism whereby meconium passed into the amniotic fluid as a reaction to fetal stress may be aspirated into the tracheobronchial tree prior to labor and birth.66 Coupled with case reports of stillborn neonates (who obviously never took a breath outside the uterus) with meconium present in their airways on postmortem examination, the conclusion was that not all meconium aspiration occurred during delivery and therefore was preventable.67–69
These observations led to several studies that critically examined the dogma surrounding management of the meconium-stained neonate. In 2000, Wiswell et al found that intubation and suctioning of vigorous neonates with MSAF produced no benefit.70 Halliday’s review in 2001 reaffirmed that no significant improvement in the rate of MAS occurs with intubation and tracheal suctioning.71 Subsequently, Vain and colleagues conducted a study of oropharyngeal suctioning on the perineum of neonates born through MSAF and found no reduction in the incidence of MAS, need for intubation, or mortality in affected neonates.72 The current recommendations by the NRP for management of the neonate born through MSAF are as follows3:
1. If the newborn is vigorous (effective respiratory effort, adequate muscle tone or active movement, HR above 100 beats per minute [BPM]), clear the mouth and nose of secretions and dry, warm, and stimulate the infant.
2. If the newborn is NOT vigorous (absent respirations or gasping, floppy or poor muscle tone, HR below 100 BPM):
a. Suction the mouth using a 12- or 14-French suction catheter.
b. Intubate the trachea with an appropriate size ETT.
c. Connect the hub of the ETT to suction using a meconium aspirator.
d. Apply suction and slowly withdraw the ETT.
e. Repeat intubation as necessary until either the amount of meconium returned is greatly reduced or the neonate’s HR falls to a level indicating additional steps in resuscitation should be carried out.
Although the studies to date indicated that depressed infants born through MSAF are at risk of developing MAS, no prospective, randomized, controlled, sufficiently powered study of endotracheal intubation and suctioning in depressed infants born through MSAF was completed at the time that this chapter was prepared.73 Thus, the optimal treatment of the neonate born through MSAF remains unknown.
Naloxone for Opioid-Induced Respiratory Depression
Naloxone is an opioid antagonist that can be administered intravenously, intramuscularly, or intratracheally. Indications for its use include respiratory depression in a neonate exposed to opioids less than 4 hours prior to delivery and born to a mother without a history of narcotic dependency. Despite this indication, there are many reasons why naloxone has been removed by the NRPSC from the short list of medications to be used for neonatal resuscitation in the DR.74,75 There is no way to ensure that the maternal history is accurate regarding use/abuse of licit/illicit substances; therefore, the neonate’s in utero exposure to narcotics cannot be unequivocally ascertained. Delivery of naloxone may initiate signs of withdrawal, including seizures in neonates who have been chronically exposed to opioids in utero.76 Because the half-life of naloxone is relatively short (approximately 60 minutes) compared to most narcotics, it is possible that as naloxone is metabolized postnatally the effects of the narcotic may once again produce respiratory depression and require repeat resuscitation.77
Respiratory depression has many causes and naloxone may not be indicated; therefore, delivery of naloxone may distract the team from performing other needed interventions and considering/treating other causes. Persistent respiratory depression should be treated with PPV to maintain the HR in the normal range, and the patient should be monitored continuously (eg, with an oximeter and frequent checks by a clinician) for a period of 24 to 48 hours after consistent spontaneous respirations return.
Cardiac Resuscitation
Bradycardia
Bradycardia is the most common dysrhythmia in the neonatal period. It is defined as a HR less than 100 BPM, although some healthy, full-term neonates may have resting HRs in the 70- to 100-BPM range with normal perfusion and hemoglobin oxygen saturation levels, especially at rest or during sleep. As a primary cardiac problem, it is seen in cases of congenital heart disease where the conduction fibers may be malformed or aberrant (as in atrioventricular septal defects, ventricular inversion, or heterotaxy syndromes) and in maternal autoimmune diseases, such as lupus, for which maternal autoantibodies cross the placenta and damage the fetal cardiac conduction system. However, the most common cause of neonatal bradycardia is hypoxia and the resultant acidosis caused by inadequate oxygen content and oxygen delivery; this may occur in utero or postnatally. In these instances, establishing adequate oxygen delivery to the myocardium is the primary goal.
HR should be assessed at 30 seconds of life; if less than 100 BPM, PPV is initiated, and the HR should be rechecked at 1 minute of life (see Figure 8-1). If at any time after PPV has been initiated the HR is found to be less than 60 BPM, CC should be initiated to augment the intrinsic cardiac output. The chest should be compressed to one-third the depth in the anteroposterior diameter using the 2-handed technique. With this technique, HCPs encircle the newborn’s thorax with both hands, thumbs side by side on the lower half of the sternum and the other 8 fingers interwoven underneath the patient’s back.78–83 The thumbs should never be completely lifted off the chest during compressions to avoid subsequent misplacement. CC and PPV are to be coordinated, delivered in a ratio of 3 compressions to 1 breath every 2 seconds for a total of 4 interventions every 2 seconds.84 If the underlying etiology of the bradycardia is felt to be cardiac rather than pulmonary in nature, a compression-to-breath ratio of 15:2 may be used.
When neonatal bradycardia is unresponsive to PPV and CC, epinephrine (EPI) is administered. EPI is an inotropic and chronotropic agent, producing increased myocardial contractility and increased HR, resulting in improved coronary artery perfusion pressure, better blood flow to the myocardium, and enhanced odds for the return of spontaneous circulation.85 EPI comes in 2 concentrations, 1:10,000 (1 g/10,000 mL = 0.1 mg/mL) and 1:1000 (1 g/1000 mL = 1 mg/mL); the 1:10,000 concentration is recommended for use in the neonatal population. The intravenous (IV) route is preferred, and achieving intravenous access should be a priority for any neonate requiring EPI; this is typically achieved in an emergency situation in the DR by inserting a catheter 2 to 4 cm into the umbilical vein or via the intraosseous (IO) route using an intraosseous needle or mechanical drill.86–88 The recommended intravenous/intraosseous dose is 0.1–0.3 mL/kg (0.01–0.03 mg/kg) of 1:10,000 EPI given every 3 to 5 minutes. If intravenous access is delayed, it is reasonable to deliver intratracheal (IT) EPI at a dose of 0.5–1 mL/kg (0.05–0.1 mg/kg) of 1:10,000 EPI. The efficacy of intratracheal EPI is dubious, likely limited by dilution in alveolar fluid, inadequate absorption because of poor/absent pulmonary blood flow during bradycardia/asystole, and pulmonary vasoconstriction secondary to acidosis.89–92
The use of volume (crystalloid and colloid solutions) in the newborn in the DR should in general be restricted to when clinical signs consistent with hypovolemia (pallor, diminished pulses, poor perfusion), possibly accompanied by a history of likely fetal blood loss (eg, cord laceration), are seen in a neonate who is unresponsive to other resuscitative measures.3 Delivery of volume without clinical indication is to be avoided to minimize the risk of a decrease in stroke volume as left ventricular end-diastolic pressure rises based on the Frank-Starling mechanism. The choice of volume should be based on overall effectiveness at reestablishing circulating intravascular volume, risk of infectious or immune-mediated sequelae, cost, and general availability.
Because of these factors, crystalloid solutions (normal saline, lactated Ringer’s) are used most frequently in emergency situations in the DR.93–99 Isotonic crystalloid solutions are equivalent to colloid solutions (whole blood, packed red blood cells, fresh frozen plasma, human serum albumin) in terms of their ability to produce short-term increases in intravascular volume and blood pressure. The recommended dose is 10 mL/kg, repeated as necessary to achieve adequate perfusion.3 Once perfusion and blood pressure are normalized, blood products can be given to treat specific deficits, such as anemia. If fetal blood loss is felt to be likely (as indicated by a sinusoidal fetal HR tracing), non-crossmatched type O Rh-negative packed red blood cells may be ordered so that they can be infused in the DR.
Tachycardia
Neonatal tachycardia is most commonly seen in the context of maternal fever and chorioamnionitis or neonatal sepsis; treatment is not directed at the heart but rather consists of performing an appropriate workup for sepsis and initiation of antibiotic therapy. Tachycardia may also be caused by anemia secondary to acute or chronic causes; associated signs include pallor, poor perfusion, and hypotension. Once again, therapy is directed not at the heart but at volume replacement with crystalloid or colloid. Tachycardic dysrhythmias secondary to intrinsic cardiac disease are typically supraventricular in nature. The resuscitation team will need to determine whether cardiac output is compromised to the extent that such a dysrhythmia requires immediate treatment in the DR at the time of birth or can await a comprehensive evaluation (electrocardiogram [ECG], echocardiogram, and consultation with a pediatric cardiologist) and targeted therapy in the NICU.
An example of a condition that may require extensive treatment immediately after birth is long-standing (weeks to months) fetal tachycardia resulting in hydrops fetalis. Initial therapy is directed at achieving adequate oxygenation and ventilation with PPV; drainage of any pleural effusions, ascites, or pericardial effusions that compromise cardiopulmonary function; and establishing intravenous access for drug and volume delivery. Therapy with vagal maneuvers, medication such as adenosine, or cardioversion may then be indicated.
Monitoring Heart Rate
Studies performed in the real clinical environment and those completed in highly realistic simulated environments raise serious questions about the ability of HCPs (even those who are highly experienced) to accurately determine HR in a neonate at the time of birth, regardless of technique (auscultation of the precordium or palpation of umbilical artery pulsations at the umbilical stump).100,101 In addition, it has been shown that inaccuracy in HR determination results in errors of commission (performing interventions not indicated) and errors of omission (lack of indicated interventions) that may result in patient harm.102 Oximetry provides an indication of pulse rate in addition to SpO2, but it often requires a minute or more to display its first signal and fails to produce a signal when perfusion is poor or nonexistent.103,104 ECG leads provide the quickest, most accurate, and most reliable means of determining neonatal HR in the DR and are likely to be used with increasing frequency, especially in low birth weight neonates, when accuracy of HR detection is a high priority.
Special Resuscitation Situations in the Delivery Room
There are a number of neonatal disease states presenting at birth that may complicate efforts at resuscitation and reduce the chance for a good clinical outcome (see Table 8-5). Although a detailed discussion of each of these conditions is beyond the scope of this chapter, it can be stated that, despite their unique nature, a general approach focusing on establishing an adequate airway, initiating effective breathing, and ensuring adequate cardiac output is indicated.
Table 8-5 Special Resuscitation Situations in the Delivery Room