Hypotension
EPIDEMIOLOGY
Incidence and Definitions
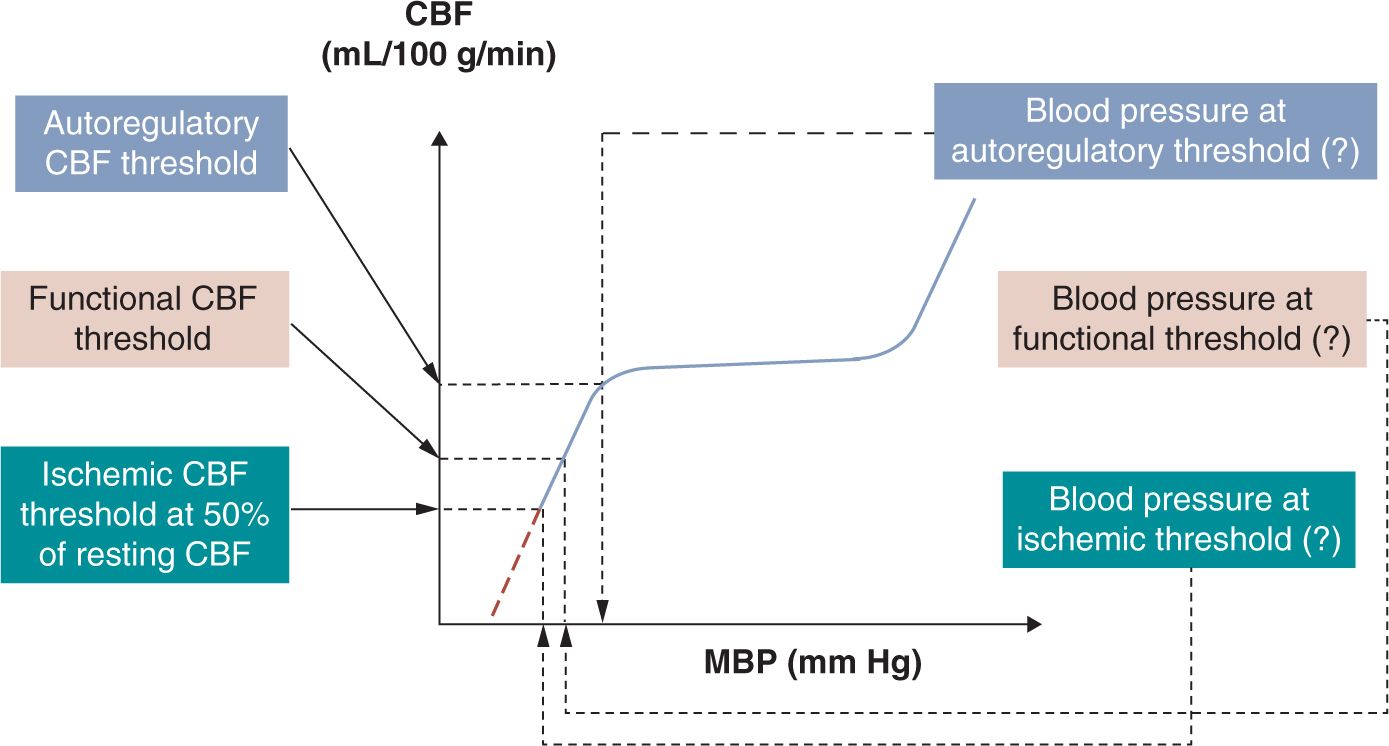
Hypotension is a common problem in the neonatal intensive care unit (NICU).1 More than half of neonates admitted to the NICU also carry the diagnosis of hypotension. Although the incidence is higher with lower gestational age at birth, the exact incidence is not known. This is primarily because of the lack of consensus on what constitutes hypotension.2 Indeed, there are many ways to define neonatal hypotension, and the differences among the definitions obviously affect the reported incidence of hypotension and the decision to treat the condition.3,4 As the ultimate goal is to ensure that oxygen delivery matches tissue oxygen demand in all organs, hypotension best can be defined based on physiologic principles, that is, by assessing the effects of decreased perfusion pressure on organ blood flow and oxygen delivery. However, because in the first, “compensated” phase of shock, vital organ (brain, myocardium, adrenal glands) blood flow and blood pressure (BP) are maintained by the neuroendocrine mechanism-driven redistribution of blood flow from nonvital organs, hypotension only presents when shock enters its second, “uncompensated” phase and vital organ blood flow also declines. Therefore, hypotension can be best defined as the BP below which vital organ (eg, brain) blood flow autoregulation is lost and cerebral blood flow (CBF) starts decreasing in proportion to the decrease in BP (the so-called autoregulatory threshold of hypotension; Figure 20-1).5 On further decrease in BP and oxygen delivery, cellular function cannot be appropriately maintained, but structural integrity is not yet significantly affected (the so-called functional threshold of hypotension; Figure 20-1).5 Finally, on further decline in BP and oxygen delivery, structural integrity of tissues becomes affected, resulting in permanent organ damage (the so-called ischemic threshold of hypotension; Figure 20-1).5 Unfortunately, there are few data on the cutoff values, and because the capacity of the cardiovascular and neuroendocrine systems to appropriately compensate is affected by gestational and postnatal age as well as the underlying pathophysiology, these values likely vary in the individual patient and even for the same patient at different times.
FIGURE 20-1 Definition of hypotension by 3 pathophysiologic phenomena5 of increasing severity: autoregulatory, functional, and ischemic thresholds of hypotension. The mean blood pressure (MBP) associated with the loss of cerebral blood flow (CBF) autoregulation is the generally accepted definition of hypotension (autoregulatory blood pressure threshold). Preliminary data suggest that the autoregulatory blood pressure threshold might be around 28 to 30 mm Hg in the very low birth weight (VLBW) neonate during the first postnatal days.5 However, at present it remains unclear what to do with this information in clinical practice. If blood pressure continues to fall, it reaches a value at which cerebral function becomes compromised (functional blood pressure threshold). Available preliminary data suggest that the functional blood pressure threshold might be around 22 to 24 mm Hg in the VLBW neonate during the first postnatal days.5 Finally, if blood pressure decreases even further, it reaches a value at which structural integrity becomes compromised (ischemic blood pressure threshold). Findings in immature animals suggest that the ischemic CBF threshold is around 50% of the resting CBF. However, the gestational and postnatal age-dependent ischemic blood pressure is not known for the preterm or term neonate. Finally, it is of note that the forebrain in general and certain forebrain structures in particular are more vulnerable than the structures of the hindbrain. See text for details. (Modified from McLean et al.5)
On the other hand, the clinical definition of hypotension is straightforward because it is absolutely arbitrary. In clinical practice, hypotension in very low birth weight (VLBW) infants has been defined as a mean BP below the gestational age in numerical value5 as this number is close to the 5th (or 10th) percentile of the population-based mean normative BP values for gestational age for the given patient population.6 A number of researchers and clinicians also define hypotension in VLBW neonates during the first postnatal days as a mean BP below 28 to 30 mm Hg.7–9 The last definition of hypotension is based on findings suggesting that the lower elbow of the CBF autoregulatory curve is around these values in VLBW neonates (Figure 20-1).9–11
More recently, likely because of the lack of a clinically relevant (ie, mortality and long-term outcome-based) definition of hypotension, the idea of “permissive hypotension” has been introduced in the literature.12 The permissive hypotension strategy calls for disregard of BP in the clinical assessment of the patient’s cardiovascular status. Instead, the strategy calls for focusing only on assessment of adequacy of organ perfusion by evaluating clinical and laboratory indicators of tissue perfusion. Although BP is the dependent variable among the factors defining macrohemodynamics (see the section on pathophysiology), appropriate perfusion pressure is absolutely necessary to drive blood flow through the entire circulatory system (macro- and microcirculations). Therefore, the idea to discard BP as one of the hemodynamic factors in the assessment of the cardiovascular status essentially disregards the basic principles of cardiovascular physiology.
In addition, the clinical and laboratory indicators of adequacy of perfusion are either unreliable or delayed in presentation, rendering them less useful.13 However, as none of the definitions of hypotension in itself has been shown to be associated with improved outcomes when “hypotension” is treated, the BP values are indeed arbitrarily used to trigger initiation of treatment. Finally, to make matters worse (ie, even more complex), having a BP above the cutoff value also does not ensure adequacy of perfusion in part because patients first enter the compensated phase of shock where BP remains within the “normal range” (see also the BP and blood flow interaction discussion that follows).
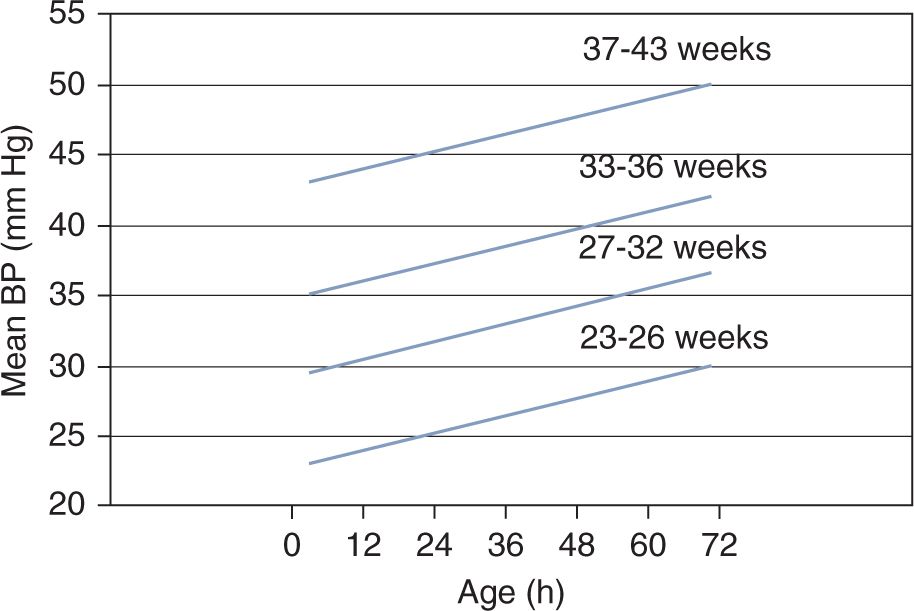
Figure 20-2 shows an example of statistically defined normal BP.14 The lines represent the lower limit of the 80% confidence interval of BP for each gestational age group during the first 3 days of postnatal life. Therefore, 90% of neonates will have a mean BP value at or above this lower limit. Although adequacy of organ perfusion cannot be deduced from these data or similar, statistically derived normative values, the graph clearly demonstrates that BP is directly related to both gestational and postnatal age and therefore can be used as a guideline in the initial assessment of neonates with suspected hemodynamic instability.15
FIGURE 20-2 This graph depicts an example of statistically defined normal blood pressure (BP) values. The lines represent the lower limit of the 80% confidence interval of mean BP in neonates during the first 3 postnatal days. Ninety percent of neonates will have a mean BP value at or above this lower-limit confidence interval. (Modified from Nuntnarumit et al.14 See the text for details.)
Risk Factors
Certain neonatal patient populations are more likely to develop hypotension and circulatory failure and suffer from the complications associated with inadequate tissue perfusion. Prematurity, lack of exposure to antenatal steroids, chorioamnionitis, perinatal infection, perinatal depression, pre- and postnatal exposure to certain medications, respiratory distress syndrome, and persistent pulmonary hypertension are among the risk factors for developing hypotension. There are many reasons that the neonate, especially the preterm neonate, is vulnerable to the hemodynamic effects of hypotension. The immaturity of organ systems in general and the brain in particular predisposes the preterm infant to organ damage. The narrow BP range of CBF autoregulation10 and its attenuation or complete loss in situations that are all but too common (eg, hypercarbia and hypotension) in the preterm neonate can adversely affect CBF and contribute to the development of brain injury.
Recently, we have proposed that vital organ assignment might be developmentally regulated, and that in extremely preterm infants the forebrain is a nonvital organ during the first 24 to 48 hours following delivery.16 Indirect evidence in preterm neonates and studies in developing animals support this hypothesis and demonstrate that, unlike in the hindbrain, vessels in the forebrain constrict in response to hypoxia or hypoperfusion.17,18
PATHOPHYSIOLOGY
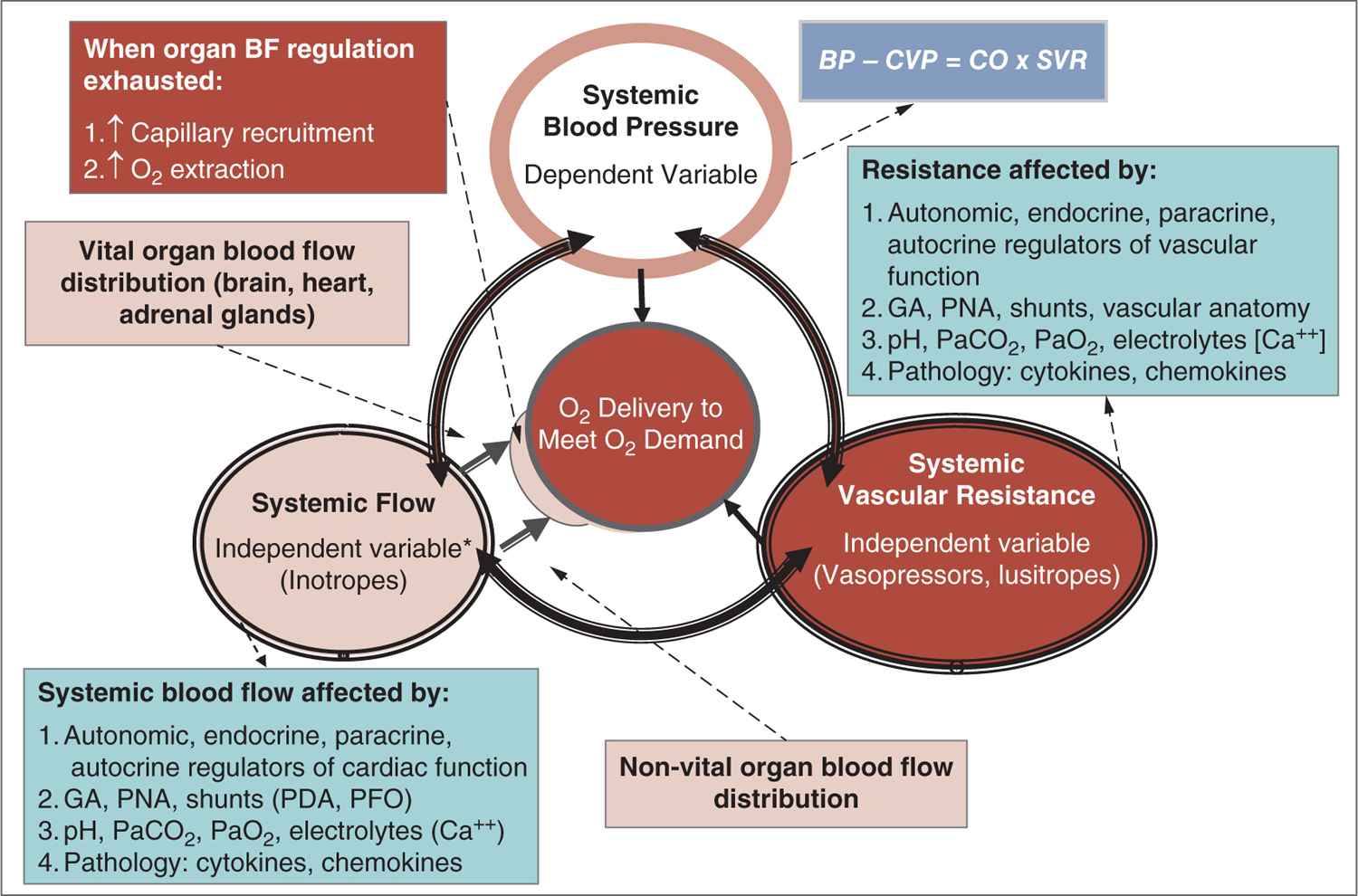
Careful attention to the principles of cardiovascular physiology and the interaction between BP and systemic and organ blood flow are important in understanding the pathophysiology of hypotension and shock (Figure 20-3).19,20 According to Poiseuille’s law, flow is directly related to the pressure gradient and diameter of the vessel and inversely related to the length of the vessel and the viscosity of the fluid. Thus, pressure is the driving force behind moving blood through the vasculature. In clinical practice, Ohm’s law is used to describe the interaction between BP, blood flow (cardiac output, CO), and systemic vascular resistance (SVR): BP ∝ SVR × CO. Accordingly, physiologic and pathologic changes in the 2 relatively independent parameters (CO and SVR) determine the dependent parameter, BP. Considering this interaction, BP would not change if, for example, CO drops by 50% and SVR doubles. This also illustrates the fact that BP is one of the principle parameters among the hemodynamic indicators of perfusion that should be monitored.
FIGURE 20-3 The major function of the circulation is to deliver oxygen and nutrients to the tissue to meet metabolic demand. The interaction between 2 factors, systemic flow and systemic resistance, ensures adequate oxygen delivery. These 2 relatively independent factors are regulated and controlled by autonomic, endocrine, and paracrine factors and affected by a host of other physiologic and pathologic mechanisms. The interaction between systemic flow and resistance ultimately determines blood pressure. Finally, if organ blood flow is insufficient, capillary recruitment and increase in oxygen extraction will keep the demand in check to maintain adequate oxygen delivery. BF, blood flow; [Ca++], calcium concentration; CO, cardiac output; CVP, central venous pressure; GA, gestational age; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen; PDA, patent ductus arteriosus; PFO, patent foramen ovale; PNA, postnatal age; SVR, systemic vascular resistance. (Modified with permission from Soleymani S, Borzage M, Seri I. Hemodynamic monitoring in neonates: advances and challenges. J Perinatol. 2010;30 Suppl:S38–S45.)
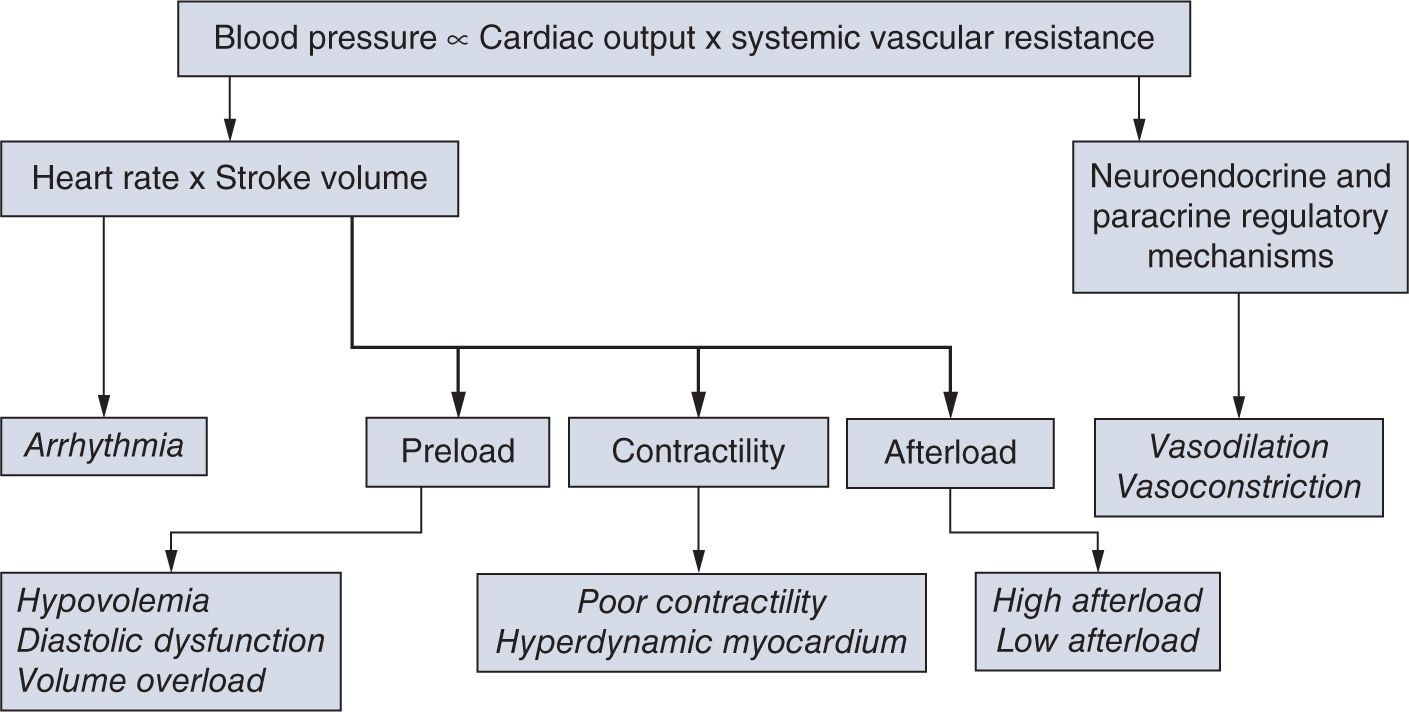
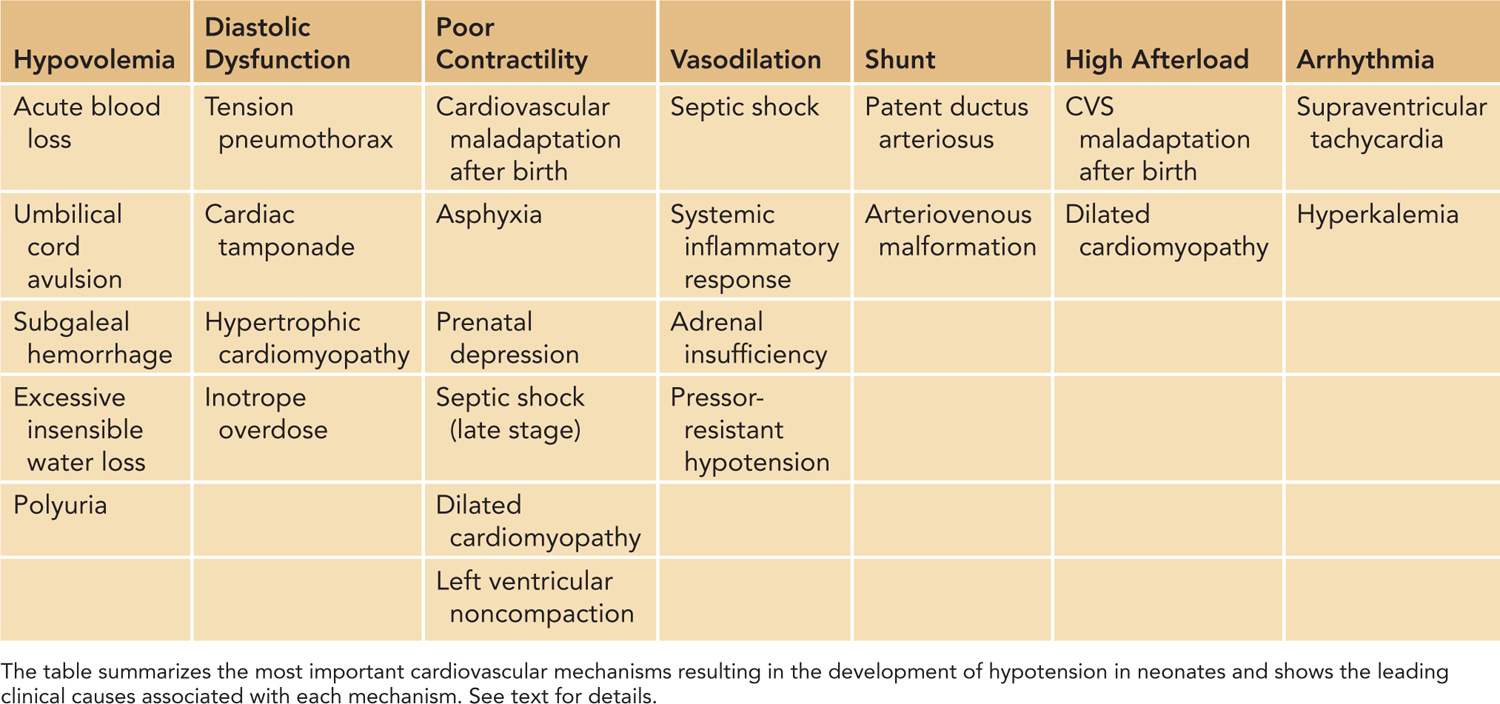
In defining the pathophysiology of hypotension and cardiovascular compromise, it is useful to evaluate the components of SVR and CO, the two so-called independent variables (Figure 20-4). Disturbances in regulation of or pathologic alterations in vascular tone, heart rate, preload, contractility, or afterload can all lead to hypotension and cardiovascular compromise. Therefore, based on the underlying pathophysiology, we can identify the primary etiology for hypotension, including disturbances of vascular tone (vasodilation and vasoconstriction); arrhythmia; conditions primarily affecting preload (hypovolemia and diastolic dysfunction); and contractility (myocardial injury or hyperdynamic myocardium) as well as increased or decreased afterload (Table 20-1). Among the underlying causes of hypotension and shock, vasodilation and poor contractility are the most common etiological factors in neonates, especially in preterm infants.
FIGURE 20-4 Blood pressure is the product of the interaction between cardiac output and systemic vascular resistance. Assessment of each component of this interaction is important and useful in identifying the underlying cause of cardiovascular compromise. (See the text for details.)
Table 20-1 Cardiovascular Mechanisms that Result in Neonatal Hypotension
Disturbances of Vascular Tone
Regulation of vascular smooth muscle tone is complex and involves a delicate balance between vasodilator and vasoconstrictor signals controlled by the autonomic nervous system as well as endocrine mechanisms and paracrine and local vasoactive factors. The most important endocrine, paracrine, and local regulators of vascular tone include nitric oxide, eicosanoids, vasopressin, catecholamines, renin-angiotensin system, and endothelin. Some of the factors, such as locally generated nitric oxide and vasodilatory eicosanoids, are increased in septic shock and generalized inflammatory diseases and are considered the main cause of the associated poor vascular tone and vasodilatory shock in these conditions. Given the high incidence of clinical conditions with elevated inflammatory mediators such as chorioamnionitis, respiratory distress syndrome, necrotizing enterocolitis, inappropriate oxygen exposure, and immaturity of the autonomic nervous system, especially in the very preterm neonate, it is not surprising that vasodilation plays a significant role in the pathogenesis of circulatory failure in these patients.
Disturbances in Heart Rate and Rhythm
Although transient sinus bradycardia and tachycardia are common in neonates, it is the more sustained problems with cardiac rhythm that can lead to hypotension and circulatory failure. Among arrhythmias potentially leading to circulatory compromise, supraventricular tachycardia and hyperkalemia-related tachyarrhythmia are the most frequently occurring clinical presentations.
Disturbances of Preload
In general, conditions that decrease preload by resulting in absolute hypovolemia, such as acute blood loss, are rare. Moreover, the lack of evidence of a well-defined relationship between blood volume and BP in preterm neonates further supports the generally accepted notion that absolute hypovolemia is not a common cause of hypotension in the neonatal period.21–23 However, it must be noted that these studies21–23 were performed on more mature preterm neonates. Moreover, the recent findings of improved hemodynamics associated with delayed cord clamping suggest that some degree of decreased circulating blood volume is indeed not uncommon, especially in the very preterm neonate, and that having a higher absolute circulatory volume at birth might be beneficial.24,25 Of course, there may be additional mechanisms in play contributing to the improved hemodynamics seen following delayed cord clamping. For example, a more gradual increase in afterload when cord clamping is delayed rather than the abrupt afterload increase associated with the immediate clamping of the cord might allow for improved adaptation of the immature myocardium of the very preterm neonate.
On the other hand, reduced preload, despite the absence of significant absolute hypovolemia, is not uncommon. High intrathoracic pressure due to an inappropriately high mean airway pressure or a tension pneumothorax as well as the presence of a pericardial effusion can significantly decrease CO by decreasing venous return. Furthermore, although its role in the pathogenesis of hypotension has not been adequately studied, the decreased compliance of the immature myocardium could also limit the filling of the heart. Finally, significant diastolic dysfunction can contribute to decreased filling and thus decreased preload in patients with hypertrophic cardiomyopathy or in patients treated with inappropriately high doses of inotropes, especially dobutamine. Patients with hypertrophic cardiomyopathy are usually born to mothers with diabetes, and the small ventricular cavity and hyperdynamic myocardium result in significant reductions in preload.
Disturbances of Myocardial Contractility
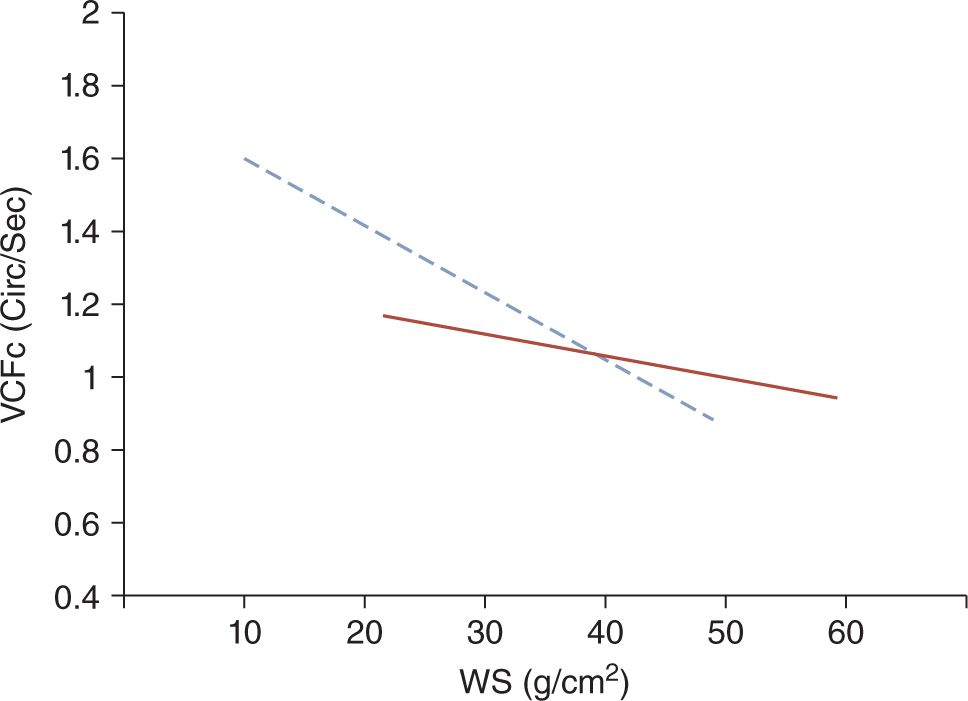
Because of both structural and functional immaturity, the myocardium of the neonate is more prone to decreased contractility (Figure 20-5).26 Myocardial dysfunction has been documented in about one-third to a half of very preterm infants presenting with hypotension or poor perfusion.27,28 It has been postulated that the higher sensitivity of the immature myocardium to afterload compared to infants, children, and adults results in poor contractility in preterm, especially very preterm, neonates. Accordingly, the abrupt increase in left ventricular afterload following the sudden removal of the low-resistance placental vascular bed at the time of cord clamping is thought to be one of the primary causes of myocardial dysfunction in very preterm neonates in the immediate postnatal period.
FIGURE 20-5 The solid line represents the normal inverse relationship between myocardial contractility (VCFc) and the afterload (WS) in children. The dotted line depicts this regression line in neonates. Because the slope of the regression line is steeper in neonates compared to older children, with an increase in afterload, the contractility of the immature myocardium of neonates decreases more rapidly than that of the mature heart of older children. (Modified from Rowland and Gutgesell.26 See the text for details.)
Another major cause of myocardial dysfunction is hypoxic-ischemic injury associated with perinatal depression.29–31 In this setting, the compensatory vasoconstriction may mask the impending circulatory failure by maintaining BP in the perceived normal range. Therefore, assessment of myocardial function by echocardiography has been recommended in all patients with low Apgar scores or evidence of perinatal depression.32
Disturbances of Afterload
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree