Crohn’s Disease
Thomas T. Sato
Subra Kugathasan
Medical College of Wisconsin, Division of Pediatric Surgery, Children’s Hospital of Wisconsin, Milwaukee, Wisconsin 53201.
Medical College of Wisconsin and Inflammatory Bowel Disease Program, Division of Pediatric Gastroenterology, Children’s Hospital of Wisconsin, Milwaukee, Wisconsin 53201.
INTRODUCTION
Inflammatory bowel disease includes the similar but distinct entities of Crohn’s disease, ulcerative colitis, and in some cases, indeterminate colitis. Inflammatory bowel disease is characterized by chronic, idiopathic gastrointestinal (GI) inflammation with typical onset in late childhood or adolescence (1). Although inflammatory bowel disease is relatively rare in the pediatric population, the effects of the disease can be quite serious and its treatment is lifelong. The surgeon’s role in the management of inflammatory bowel disease is evolving as significant new therapeutic interventions are being introduced into clinical use (2).
Although the term inflammatory bowel disease is used for a variety of chronic idiopathic inflammatory bowel disorders, Crohn’s disease and ulcerative colitis comprise the vast majority of observed pediatric cases. Crohn’s disease is characterized by a transmural inflammatory process that can involve any region of the GI tract, whereas ulcerative colitis is a mucosal inflammatory process limited to the colon. Although there are several similarities in the manifestations of inflammatory bowel disease in children compared with adults, there are also substantial differences and unique issues that arise in the pediatric population. In particular, Crohn’s disease is more common than ulcerative colitis in children, and when ulcerative colitis is diagnosed during childhood, the finding of extensive pancolitis is not unusual. In addition, children with inflammatory bowel disease may experience significant growth impairment, pubertal delay, and difficulty with psychosocial adjustment during adolescence and early adulthood. Ulcerative colitis is discussed in detail in Chapter 87.
Epidemiology
The highest disease rates for inflammatory bowel disease are in western countries. Epidemiologic data are consistent with a gradual increase in the incidence of Crohn’s disease and ulcerative colitis since the mid-1950s (3,4). Both genetic and environmental factors appear to interact to make specific individuals susceptible to the disease (5).
Retrospective European studies and a prospective, population-based study in Great Britain have demonstrated that the incidence of inflammatory bowel disease has increased since the mid-1970s (6,7,8). Generally, there has been greater increase in the incidence of Crohn’s disease than ulcerative colitis in Europe. An initial, systematic, population-based epidemiologic study in North America has recently been completed. All children younger than 18 years of age newly diagnosed with inflammatory bowel disease in the state of Wisconsin over a 2-year period were evaluated. The incidence of inflammatory bowel disease was 7 per 100,000 children, with equivalent incidence across ethnic groups and population density. Urbanization and population density did not appear to be related to a change in the incidence of the disease. Only 11% of the patients had a family history of inflammatory bowel disease, suggesting that the pathogenesis of inflammatory bowel disease is multifactorial (9).
Etiology of Inflammatory Bowel Disease
The etiology of inflammatory bowel disease remains unknown. The chronic nature of the inflammatory process in both Crohn’s disease and ulcerative colitis suggests that there is both an initiating event, as well as factors that allow perpetuation of the GI inflammatory response. Despite the fact that Crohn’s disease and ulcerative colitis are distinct clinical entities, they are both characterized by an inflammatory response that may overlap and make definitive clinical diagnosis difficult. A widely accepted hypothesis to explain the observed clinical manifestations of inflammatory bowel disease is that an environmental trigger induces
an inflammatory response in genetically susceptible individuals (10). Following establishment of the inflammatory response, it is speculated that host genetic factors play a substantial role in the trajectory of the inflammatory process. Given the broad clinical spectrum of inflammatory bowel disease, it is likely that there are several genetic loci that lead to host susceptibility, as well as different patterns in the host inflammatory response.
an inflammatory response in genetically susceptible individuals (10). Following establishment of the inflammatory response, it is speculated that host genetic factors play a substantial role in the trajectory of the inflammatory process. Given the broad clinical spectrum of inflammatory bowel disease, it is likely that there are several genetic loci that lead to host susceptibility, as well as different patterns in the host inflammatory response.
There is substantial evidence that specific genes may influence the development and the progression of inflammatory bowel disease. The NOD2/CARD15 gene is associated with Crohn’s disease. In particular, mutations of the NOD2/CARD15 gene may contribute to the phenotypic expression of inflammatory bowel disease in children with early onset of disease and/or with a strong family history of Crohn’s disease (observed in the Ashkenazi Jewish population) (11,12). As many as 17% to 40% of patients with Crohn’s disease may have mutation(s) in the NOD2 gene, which is believed to regulate the inflammatory response to exposure to bacterial lipopolysaccharide (13). Genetic predisposition to an altered or deficient response to bacterial lipopolysaccharide in the intestinal lumen may be one mechanism that makes specific individuals more susceptible for the development of Crohn’s disease. The use of genetic information may allow estimation of an unaffected person’s risk of developing inflammatory bowel disease, the expected disease progression of an already affected individual, and specific targeting of therapy based on pharmacogenetic principles.
The triggering event or agent that initiates inflammatory bowel disease in a genetically susceptible individual is of considerable interest. The GI mucosal barrier is a highly regulated and immunologically active surface with constant inflammatory challenge from bacteria. Involved colonic mucosa from patients with established inflammatory bowel disease has been observed to permit significantly more bacterial adhesion compared with specimens from healthy subjects (14). Intact mucosa in adjacent areas of bowel appears to maintain barrier function against bacterial invasion, suggesting that mucosal changes in inflammatory bowel disease may compromise the barrier function. Once this barrier is compromised, expansion of the gut-associated lymphoid tissue, including the gut intraepithelial lymphocytes, occurs rapidly. Although the host inflammatory response is essential in the regulation of the GI tract, uncontrolled or persistent activation of the intestinal inflammatory response by elements of normally occurring bacterial flora may, in part, be responsible for the manifestations of the disease. Several studies have failed to identify a single infectious agent that is responsible for the initiation and establishment of the disease. In addition, there are currently no compelling data to suggest that selective decontamination of the GI tract is an effective preventive strategy for inflammatory bowel disease.
DIAGNOSIS
Clinical Presentation
A detailed history and physical examination remain the most important aspects in the evaluation of an adolescent with abdominal pain suspected of having inflammatory bowel disease. The clinical presentation of Crohn’s disease varies with the anatomic location(s) of involvement, which can occur in any region of the GI tract. In comparison, the clinical presentation of ulcerative colitis is relatively uniform given that the primary disease is limited to the colon. Table 84-1 summarizes the presenting clinical symptoms and signs in Crohn’s disease and ulcerative colitis in children. In general, the severity of GI inflammation reflects the severity of the clinical presentation.
Abdominal Pain
The most common presenting complaint of children and adolescents with inflammatory bowel disease is abdominal pain (2,15). Most children and adolescents ultimately diagnosed with inflammatory bowel disease do not present with the acute onset of abdominal pain; however, upon careful review, there is usually a history of recurrent or persistent pain over time. The pain may not be specific or
well localized. Establishment of a diagnosis on the basis of symptoms and abdominal examination may be difficult. Given the pathophysiology, a high index of suspicion must be maintained when a child presents with a history of subacute or chronic abdominal pain. Abdominal pain associated with Crohn’s disease tends to be severe and persistent, and may either awaken the adolescent from sleep or interfere with normal eating patterns. Involvement of the terminal ileum or ileocecal region with Crohn’s disease is associated with a history of chronic or recurrent right lower quadrant abdominal pain that may mimic acute appendicitis in all aspects except symptom duration. A palpable mass may be present in the right lower quadrant secondary to an inflammatory phlegmon. In 10% of pediatric patients with Crohn’s disease, odynophagia and dysphagia may be observed with esophageal involvement (15).
well localized. Establishment of a diagnosis on the basis of symptoms and abdominal examination may be difficult. Given the pathophysiology, a high index of suspicion must be maintained when a child presents with a history of subacute or chronic abdominal pain. Abdominal pain associated with Crohn’s disease tends to be severe and persistent, and may either awaken the adolescent from sleep or interfere with normal eating patterns. Involvement of the terminal ileum or ileocecal region with Crohn’s disease is associated with a history of chronic or recurrent right lower quadrant abdominal pain that may mimic acute appendicitis in all aspects except symptom duration. A palpable mass may be present in the right lower quadrant secondary to an inflammatory phlegmon. In 10% of pediatric patients with Crohn’s disease, odynophagia and dysphagia may be observed with esophageal involvement (15).
TABLE 84-1 Presenting Clinical Symptoms and Signs of Children Diagnosed with Crohn’s Disease and Ulcerative Colitis. | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Diarrhea
The vast majority of children and adolescents with inflammatory bowel disease present with diarrhea at some point in their clinical course (15). Whether from Crohn’s disease or ulcerative colitis, involvement of the colon may often be associated with grossly bloody diarrhea. With distal colonic involvement, the diarrhea may be characterized by urgency, tenesmus, and incontinence, and is often nocturnal. Crohn’s disease involving only the small bowel may also present with episodic diarrhea (16,17). In conjunction with abdominal pain, children with significant, persistent diarrhea may become more socially isolated, particularly in school where frequent trips to the restroom are either discouraged or embarrassing.
Hematochezia
The presence of hematochezia suggests colonic involvement. Adolescents with Crohn’s disease of the small intestine are less likely to present with rectal bleeding than individuals with ulcerative colitis (18,19). As many as one-half of all children with inflammatory bowel disease present with relatively mild symptoms, characterized by less than four stools per day and intermittent hematochezia (20). Stools may have only streaks of blood, or they may only be hemoccult positive. Approximately 30% of children with either Crohn’s disease or ulcerative colitis are moderately ill, presenting with gross hematochezia associated with frequent diarrhea. In 10% to 15% of cases of pediatric inflammatory bowel disease, patients present with acute, fulminant disease characterized by greater than six episodes of diarrhea per day with copious hematochezia that may require red blood cell transfusion. Fulminant colitis is a potentially life-threatening event that may occur in Crohn’s colitis, ulcerative colitis, or indeterminate colitis. Clinical presentation of fulminant colitis includes abdominal pain, nausea, anorexia, and bloody diarrhea associated with systemic manifestations of tachycardia, hypotension, and diffuse abdominal distention and tenderness. Toxic megacolon, although rare in the pediatric age group, is a complication of inflammatory bowel disease that is a surgical emergency requiring prompt total abdominal colectomy and end ileostomy (21).
Weight Loss and Growth Impairment
As many as 80% of children and adolescents with Crohn’s disease will have weight loss prior to definitive diagnosis (15,22). Weight loss is a more common finding in Crohn’s disease than in ulcerative colitis in the pediatric population. In some children with Crohn’s disease, the presenting clinical finding is linear growth deceleration (23). Deviation from growth velocity curves and height for age curves may precede the diagnosis of Crohn’s disease, even in the absence of proven nutrient malabsorption or reduced caloric intake. In children with established Crohn’s disease, the resting energy expenditure is generally normal (24). It is unknown why growth impairment occurs, although lack of adequate caloric intake secondary to abdominal pain, diarrhea, or anorexia may reflect active inflammatory disease, growth hormone resistance, or medication effects. Because the pubertal growth spurt accounts for approximately 16% of the adult height, disease onset in prepubertal children prior may have a substantial impact on total linear growth. Overall, the prevalence of significant growth impairment during childhood and adolescence in pediatric patients with inflammatory bowel disease is approximately 13% to 58% (18,25).
Delayed Puberty
Similar to deceleration of linear growth, the presence of inflammatory bowel disease in prepubertal children is associated with significant delay in puberty (26,27). Delayed or prolonged duration of puberty is more common in children with Crohn’s disease than ulcerative colitis, particularly in children who have active disease without a history of remission, or in children with frequent disease exacerbations during the prepubertal period. Prepubertal onset of Crohn’s disease has been reported to be associated with a delay in menarche until 16 years of age or older in 73% of girls; in comparison, girls with ulcerative colitis in this same study achieved puberty at age 14 years or younger (27). The delay in sexual maturation is multifactorial and is believed, in part, to reflect malnutrition. In some children with active Crohn’s disease associated with growth arrest, supplemental caloric intake may be useful to promote puberty onset and increased growth velocity (28). Adequate caloric intake alone does not guarantee appropriate progression through puberty and increased growth velocity for all children with active inflammatory bowel disease, suggesting that there are other factors
contributing to delayed sexual maturation. In adolescents with delayed puberty secondary to active Crohn’s disease, intestinal resection of the active inflammatory bowel has been associated with progression of puberty within 1 year of operative intervention (29). It is unknown why intestinal resection leads to progression of puberty in this population, but it is speculated that the inhibitory effects of inflammatory mediators such as tumor necrosis factor-alpha and interleukin-1 are reduced with removal of the inflammatory focus. Because both nutritional and inflammatory mediators can exert effects on the hypothalamic-pituitary-gonadal axis, decreased caloric intake and reduced body weight are associated with decreased activity of the hypothalamic neurons that produce gonadotropin releasing hormone. This can lead to delayed gonadal maturation and subsequent delay in puberty as observed in prepubertal children or adolescents with inflammatory bowel disease.
contributing to delayed sexual maturation. In adolescents with delayed puberty secondary to active Crohn’s disease, intestinal resection of the active inflammatory bowel has been associated with progression of puberty within 1 year of operative intervention (29). It is unknown why intestinal resection leads to progression of puberty in this population, but it is speculated that the inhibitory effects of inflammatory mediators such as tumor necrosis factor-alpha and interleukin-1 are reduced with removal of the inflammatory focus. Because both nutritional and inflammatory mediators can exert effects on the hypothalamic-pituitary-gonadal axis, decreased caloric intake and reduced body weight are associated with decreased activity of the hypothalamic neurons that produce gonadotropin releasing hormone. This can lead to delayed gonadal maturation and subsequent delay in puberty as observed in prepubertal children or adolescents with inflammatory bowel disease.
Perirectal Disease and Fistula
Perirectal and perianal disease, along with fistula formation, are unique findings in Crohn’s disease. In children with Crohn’s disease and perianal or perirectal disease, approximately 25% to 30% will have multiple anal skin tags, nonhealing anal fissures, perianal or perirectal fistulas, or perirectal abscess (30). Perirectal pain is unusual unless there is an abscess present, and suspicion for an abscess should be high in the presence of erythema, tenderness, and swelling in association with systemic symptoms such as fever. Crohn’s disease should be considered in any child with recurrent, multiple, or persistent perianal abscesses or fistulas. In addition, Crohn’s disease should be suspected in children with a history of weight loss, abdominal pain, diarrhea, and perianal disease. Rarely, perianal or perirectal involvement secondary to chronic granulomatous disease or tuberculosis may be encountered (31,32). A small percentage of adolescents may have aggressive and highly destructive perianal Crohn’s disease as their predominant symptom (33), although multiple perianal and perineal fistula observed in adults with a “watering can” perineum are fortunately rare in the pediatric population. Significant perianal involvement with Crohn’s disease in the pediatric population may be clinically misdiagnosed as nonaccidental traumatic injury secondary to sexual assault (34).
The most common fistulas encountered in Crohn’s disease are enterocutaneous and comprise at least 80% of the clinical fistulas observed. Fistulas may occur between small and large intestine, vagina, bladder, and abdominal
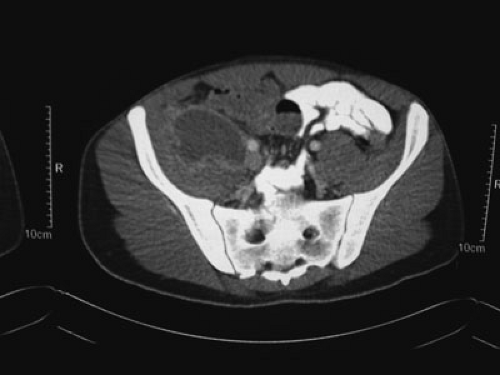
wall. Fistulas may present as localized drainage of enteric contents from the abdominal wall, perineum, or operative wound. Enteroenteric or enterocolic fistulas may present with malnutrition secondary to malabsorption. Patients with Crohn’s disease presenting with a newly diagnosed intraabdominal, pelvic, or psoas abscess should be assumed to have a transmural fistula (Fig. 84-1).
wall. Fistulas may present as localized drainage of enteric contents from the abdominal wall, perineum, or operative wound. Enteroenteric or enterocolic fistulas may present with malnutrition secondary to malabsorption. Patients with Crohn’s disease presenting with a newly diagnosed intraabdominal, pelvic, or psoas abscess should be assumed to have a transmural fistula (Fig. 84-1).
Clinical features distinguishing Crohn’s disease from ulcerative colitis are listed in Table 84-2.
TABLE 84-2 Clinical Features Distinguishing Crohn’s Disease and Ulcerative Colitis. | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
EXTRAINTESTINAL MANIFESTATIONS
Established inflammatory bowel disease is often (25% to 35%) accompanied by diverse extraintestinal manifestations that can affect virtually any anatomic systems. The most common extraintestinal findings in children are involvement of the dermatologic, rheumatologic, ocular, and hepatobiliary systems. Severity of the extraintestinal manifestation is not necessarily related to the severity of inflammatory bowel disease, and it is uncommon for an extraintestinal manifestation to be the singular presenting event in children. These manifestations are observed in both Crohn’s disease and ulcerative colitis.
Arthritis/Arthralgia
Symptoms related to arthritis, including ankylosing spondylitis or sacroilitis, and peripheral arthropathy (knees, ankle, hips, wrists, and elbows) have been well-described as coexisting or even preceding the development of GI symptoms of inflammatory bowel disease (35). Arthritis is more commonly observed with colonic involvement, and the severity of arthritic symptoms may be independent of the extent or severity of the inflammatory bowel disease. Arthritic symptoms may be transient and migratory in presentation. Clubbing of the fingers in children with Crohn’s disease reflects the progression of hypertrophic osteoarthopathy of the distal finger joints.
Skin and Oropharyngeal Mucosa
A characteristic dermatologic finding in children or adolescents with inflammatory bowel disease is erythema nodosum or pyoderma gangrenosum. Erythema nodosum is an inflammatory lesion characterized by a raised, erythematous, painful nodule occurring over the tibia, lower leg, ankle, or extensor surfaces of the arms. Pyoderma gangrenosum is a small, painful skin pustule that coalesces into a larger, sterile abscess, forming a necrotic, deep, transdermal ulcer. The association between these skin lesions and the presence of inflammatory bowel disease is significant enough that children diagnosed with either skin lesion should undergo a complete GI workup even in the absence of GI symptoms (15).
Examination of the mouth and oropharynx is important in children and adolescents suspected of having inflammatory bowel disease. Aphthous ulcerations of the mouth or gums may accompany intestinal disease. In Crohn’s disease, granulomatous inflammation of the lips, gums, and face have been described (36).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree