Common Problems and Complications of Premature and Full-term Infants
Jeffrey Dawson
Joan Rosenbaum
Karen Wickline
Sherrie M. Hauft
Amit Mathur
Aaron Hamvas
Anna Lijowska
Claudia Gerard
F. Sessions Cole
Department of Pediatrics, Division of Newborn Medicine, Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, Missouri 63110.
Department of Pediatrics, Division of Newborn Medicine, Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, Missouri 63110.
Department of Pediatrics, Division of Newborn Medicine, Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, Missouri 63110.
Department of Pediatrics, Division of Newborn Medicine, Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, Missouri 63110.
Department of Pediatrics, Division of Newborn Medicine, Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, Missouri 63110.
Department of Pediatrics, Division of Newborn Medicine, Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, Missouri 63110.
Department of Pediatrics, Division of Newborn Medicine, Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, Missouri 63110.
Department of Pediatrics, Division of Newborn Medicine, Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, Missouri 63110.
Department of Pediatrics, Division of Newborn Medicine, Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, Missouri 63110.
COMMON PROBLEMS IN THE PREMATURE INFANT
Epidemiology of Preterm Birth
The two principal contributors to neonatal death in the United States are birth defects and preterm delivery (1,2). Despite continuing technologic advances in the care of acutely ill newborn infants that have helped lower the infant mortality rate in the United States from 26 in 1,000 live births (1960) to 6.9 in 1,000 (2000), the rate of preterm delivery of premature infants (less than 37 weeks of gestation or less than 2,500 g birthweight) has not changed significantly since 1960 and is the largest single contributor to infant mortality (2). The United States ranks between twentieth and thirtieth among countries around the world in infant mortality and premature delivery rates (3). Epidemiologic investigations have identified important population-based risk factors for premature delivery, including African American race, poverty, low maternal educational attainment, substance abuse (tobacco, cocaine, alcohol) during pregnancy, no or inadequate prenatal care (prenatal care initiated after the first trimester and fewer than five visits through 37 completed weeks of gestation), previous adverse pregnancy outcome (preterm delivery, more than two spontaneous abortions, stillbirth, or neonatal death), or multiple gestation (4). Risk of preterm delivery is twofold higher among African American women (13.0% in 2000) than among white (6.6%) or most Hispanic (6.4%) women (1).
Clinical Causes of Preterm Birth
Maternal, paternal, fetal, placental, genetic, and environmental factors may act individually or together to cause premature delivery (5). Individualized preconceptual and prenatal risk assessments are important in planning for optimal outcomes for both mother and infant. Ongoing risk assessment during pregnancy permits matching of biologic risk of mother and fetus with appropriate availability of skilled personnel, technology, and facilities.
Uterine and placental anomalies increase the risk of preterm delivery. Many women with recurrent second- and third-trimester losses have immunologic diseases (e.g., systemic lupus erythematosus or antiphospholipid antibody syndrome) that disrupt immunologic adaptation necessary for maternal tolerance to paternal antigens on fetal and placental cells. Increased uterine volume and myometrial stretching in multiple-gestation pregnancies and in pregnancies characterized by polyhydramnios suggest a potential mechanism to account for the 40% to 50% rate of preterm delivery associated with these conditions.
Nutritional deficiencies (e.g., iron and calcium) and substance abuse (e.g., tobacco) provide important opportunities for preventive interventions. Tobacco is the most significant and preventable cause of low-birth-weight infants in the United States. Cessation of smoking among all reproductive-age women in the United States would result in about a 25% reduction in the low-birth-weight rate.
Prevention of infectious processes may also significantly reduce the frequency of preterm delivery (6). The pathway between infection and preterm labor or preterm rupture of amniotic membranes may involve both weakening of amniotic membranes by bacterial products and decidual inflammation elicited by bacterial invasion.
Diagnosis of Prematurity
The diagnosis of prematurity can be based on antenatal estimation of duration of gestation or on physical characteristics of the infant noted postnatally (7). The most accurate estimates use multiple fetal measurements, are obtained during the first half of pregnancy, and are accurate within 4 days. Estimates based on third-trimester fetal ultrasound measurements have potential errors of 1.5 to 4 weeks.
Use of neuromuscular maturity, anthropometric measurements, and physical findings of the newborn infant permit postnatal estimation of gestational age (GA) (8). The Ballard examination and modified Dubowitz examination provide the most reliable postnatal estimates of gestational age.
Fetal Circulation and Patent Ductus Arteriosus
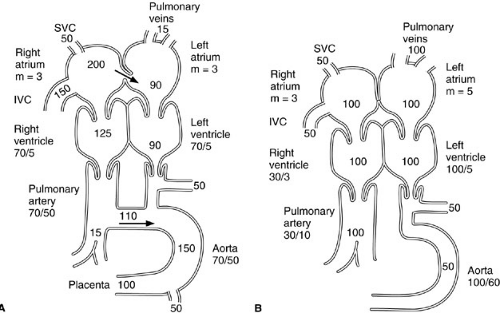
Persistent patency of the ductus arteriosus is a common problem in sick, premature infants and can lead to high-output cardiac failure due to aortic to pulmonary artery shunting. In the fetus, 69% of combined ventricular output is oxygenated blood that travels from the placenta to the fetal right atrium through the umbilical vein, ductus venosus, and inferior vena cava (Fig. 4-1). A portion of oxygen-rich blood that enters the right atrium from the inferior vena cava is shunted preferentially across the atrial septum through the foramen ovale to the left atrium. This oxygenated blood is pumped into the ascending aorta by the left ventricle and is thus more available to the coronary arteries and subclavian and carotid arteries than to the systemic circulation. Because only about 7% of fetal right ventricular output goes to the lungs, owing to supersystemic pulmonary vascular resistance during fetal life, the right ventricle performs about twice as much work as the left ventricle during fetal life. About 90% of right ventricular output flows to the systemic circulation through the ductus arteriosus. Fetal oxygen tension is usually 25 to 30 mm Hg.
The ductus arteriosus shunts blood flow from the pulmonary artery into the aorta. Within hours to days after delivery in full-term infants, as pulmonary vascular resistance falls and arterial oxygenation increases, the patent ductus arteriosus (PDA) closes by constriction of vascular smooth muscle in its wall, which shortens and narrows its lumen (9). Failure of the PDA to close after postnatal reduction in pulmonary vascular resistance results in left-to-right shunting from aorta to pulmonary artery, pulmonary hyperperfusion, high-output congestive heart failure, and a diastolic steal syndrome, which reduces perfusion to the splanchnic and renal vascular beds. Failure of closure with persistently supersystemic postnatal pulmonary vascular resistance results in right-to-left shunting from pulmonary artery to aorta and systemic hypoxia proportional to the magnitude of the pulmonic-aortic pressure gradient.
In the preterm infant, functional closure is often delayed. Clinical evidence of a hemodynamically significant PDA includes a systolic or continuous murmur, bounding pulses with widened pulse pressure, hyperdynamic
precordium, tachypnea, tachycardia, hepatomegaly, and worsening respiratory failure. Confirmation of patency can be made by echocardiography.
precordium, tachypnea, tachycardia, hepatomegaly, and worsening respiratory failure. Confirmation of patency can be made by echocardiography.
Management of these infants must be individualized. For a premature infant with a hemodynamically significant PDA, pharmacologic interruption with indomethacin should be considered. Symptoms of PDA respond to treatment with indomethacin in about 70% of infants (10). Symptoms recur in about 30% of these infants, owing to reopening of the ductus arteriosus. These infants require additional treatment with indomethacin or surgical ligation. Because of side effects of indomethacin therapy, which include inhibition of platelet function, reduction of splanchnic blood flow, bowel perforation, and reduction of renal blood flow, treatment of infants with asymptomatic PDA remains controversial (11).
Surgical interruption of the ductus arteriosus is a safe alternative to indomethacin treatment in infants unresponsive to indomethacin or in those whose clinical situation contraindicates its use (12,13). The operative mortality rate at centers where experienced surgical teams are available is less than 1%. The morbidity of the procedure is also low (12,13). Nonsurgical transcatheter closure has also been used with increasing success (13).
Hyaline Membrane Disease
Hyaline membrane disease (HMD) has a characteristic pathologic pulmonary appearance, biochemical cause, and clinical phenotype, and contributes significantly to morbidity and mortality among premature infants (14). The pathologic appearance of premature infant lungs affected by this disease includes eosinophilic, proteinaceous material that fills alveolar spaces and forms characteristic hyaline membranes. The primary biochemical abnormality in HMD is deficiency of the pulmonary surfactant, a complex mixture of phospholipid and proteins produced in alveoli by the type II pneumocyte, a pulmonary epithelial cell. The pulmonary surfactant lowers surface tension at the air–liquid interface in alveoli and thereby permits maintenance of alveolar patency at end expiration. Surfactant production is reduced by (1) an inadequate number of type II pneumocytes, (2) lack of hormonal signals for surfactant production, and (3) immaturity of type II pneumocyte synthesis or secretion of surfactant proteins or phospholipids. Reduced surfactant function can be caused by (1) inhibition by plasma proteins, plasma cholesterol, and bacterial products; (2) pulmonary hemorrhage; and (3) meconium aspiration. Reduced surfactant function leads to increased atelectasis, worsening pulmonary compliance, and progressive respiratory failure characterized by hypercarbia, hypoxemia due to ventilation-perfusion mismatch, and acidosis due to both hypercarbia and inadequate tissue oxygenation. Clinical signs of surfactant deficiency occur within the first 12 hours of life, and include tachypnea, grunting, nasal flaring, and chest retractions. As pulmonary surfactant production increases, functional pulmonary abnormalities resolve.
The diagnosis of HMD is based on clinical, biochemical, and radiographic characteristics. In addition to the clinical symptoms noted earlier, the chest radiograph shows a homogeneous, ground-glass appearance with visible air bronchograms. Biochemical evidence of surfactant deficiency may be available from antenatal amniotic fluid testing of pulmonary phospholipid maturity. A lecithin/sphingomyelin ratio of less than two, absence of detectable phosphatidylglycerol, or lack of stabilization of bubbles at an air–liquid interface (the shake test) each suggest surfactant deficiency. These tests can also be performed on gastric aspirate fluid obtained within 60 minutes of delivery or on tracheal aspirate fluid. Results of any of these tests that indicate surfactant sufficiency exclude HMD. Frequently, the clinical presentation of HMD is indistinguishable from pulmonary or systemic bacterial infection. This clinical ambiguity requires careful evaluation for infection in all infants treated for HMD and initiation of systemic antibiotic coverage until clinical, biochemical, and microbiologic testing excludes bacterial infection.
Treatment of HMD begins with a high index of clinical suspicion. Because pulmonary function in untreated infants with HMD deteriorates during the first 3 to 4 days of life, prompt initiation of surfactant replacement and positive pressure ventilation to reduce atelectasis and improve gas exchange are often indicated. The classic ventilatory strategy for infants with HMD includes positive end-expiratory pressure to maintain alveolar patency at end expiration, maintenance of adequate minute ventilation with pressure- or volume-cycled ventilators, and administration of adequate inspired oxygen to maintain oxygen delivery to tissues (15). Alternative ventilatory strategies, including jet ventilation or high-frequency oscillatory ventilation, have been useful in clinical situations characterized by onset of pulmonary interstitial emphysema or multiple pneumothoraces within the first 24 hours of life (16). Because of the rapid changes in pulmonary compliance and surfactant production that can occur during the first week of life, rigorous attention to gas exchange and ventilatory strategy must be provided to ensure potential pulmonary sequelae of HMD caused by barotrauma and oxygen toxicity are minimized.
Surfactant replacement therapy was approved for use in the United States in 1990 (17). Pharmacologic preparations with surfactant activity can be administered directly into the tracheobronchial tree through an endotracheal tube. These preparations spread rapidly to distal alveoli, reduce surface tension, and improve pulmonary compliance. Surfactant replacement may be administered to symptomatic infants within the first 12 to 24 hours of life or may be given to high-risk infants prophylactically in the
delivery room. Infants who respond to the first dose of surfactant by improved pulmonary compliance and reduced need for oxygen administration may require further doses during the first 48 hours of life.
delivery room. Infants who respond to the first dose of surfactant by improved pulmonary compliance and reduced need for oxygen administration may require further doses during the first 48 hours of life.
Although mortality from HMD has significantly decreased since introduction of oxygen administration, mechanical ventilation, and surfactant replacement therapy, prevention of prematurity through improved access to prenatal care and use of antenatal glucocorticoids to accelerate pulmonary surfactant maturation represent the two most effective strategies to improve outcomes of premature infants. This clinical benefit cannot be duplicated by postnatal administration to the newborn infant, probably because of the role of the placenta in metabolizing prenatally administered glucocorticoids to steroid derivatives that are active on fetal lung.
Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia (BPD), or chronic lung disease (CLD), in infants is the most common pulmonary sequela of premature infants. Diagnostic criteria for BPD include the need for supplemental oxygen or an abnormal chest radiograph at 28 days of age or 36 weeks postconceptual age (depending on gestational age of the infant at birth) (18). The term was originally used to describe the radiologic and pathologic changes observed in premature infants who did not recover within 4 weeks from HMD (19). BPD is now used to describe CLD that results from any condition in the neonatal period (20). Despite the diverse diseases that lead to the development of BPD, infants with BPD share clinical and pathologic characteristics.
The incidence of BPD among premature infants is inversely proportional to gestational age and birth weight. Up to 70% of infants with birth weights less than 1,000 g develop BPD. With the increased use of antenatal steroids, surfactant replacement, improved methods of ventilation, and increased attention to nutritional needs of the sick premature infant, the birth weight-specific incidence of BPD is decreasing. Improved survival of sick infants, however, has increased the size of the population at risk for developing this disease.
Many factors play a role in the development of BPD (18,20). The most significant factors are the degree of pulmonary structural and functional immaturity at birth, the degree and length of ventilatory and oxygen support, and an arrest of postnatal lung development (20,21). The chest radiograph progresses to show areas of atelectasis and cystic changes with overexpansion. More recent pathologic description suggests an arrest of alveolar development without epithelial metaplasia, smooth muscle hypertrophy, or fibrosis (18,20). These infants show signs of respiratory distress with tachypnea, retractions, wheezing, and need for supplemental oxygen. Pulmonary function testing may show increased resistance as a result of airway injury, bronchospasm, or interstitial edema. Lung compliance may also be decreased. Hypoxemia may result from ventilation-perfusion mismatch and from loss of surface area for gas exchange. Pulmonary hypertension and cor pulmonale may develop as a result of chronic hypoxemia (22).
Postnatal prevention and treatment of BPD require early identification of at-risk infants and strategies to minimize the inflammatory effects of barotrauma and oxygen toxicity. Both the reduction of ventilatory requirements and the use of surfactant replacement therapy decrease the severity of BPD. Early attention to nutritional support is critical to optimize lung growth and recovery in infants with BPD. They frequently exhibit growth failure presumably due to high energy expenditure and require high caloric density formulas.
Although the heterogeneity of BPD makes extrapolation from clinical trials to individual patients difficult, several pharmacologic interventions should be considered for infants with BPD. Once extubated, the maintenance of adequate oxygen delivery to tissues by administration of low-flow oxygen by nasal cannula is critical to optimize outcome of these infants. Because interstitial and peribronchiolar pulmonary edema plays a role in BPD, diuretics have been useful in improving lung mechanics. Electrolyte and mineral imbalances with resultant growth deficiency, nephrocalcinosis, or osteopenia that occur with chronic furosemide administration must be carefully monitored and corrected. Treatment of clinical and histologic evidence of airway reactivity with bronchodilators has benefit. The use of a corticosteroid in the treatment of BPD is no longer considered standard therapy (18,20,23).
The contribution of gastroesophageal reflux (GER) to worsening BPD must be individually assessed. Chronic aspiration can be exacerbated by oral bronchodilators such as theophylline. An initial approach to evaluation of these problems includes documentation of the effects of chronic aspiration on growth, pulmonary radiographic appearance, and respiratory compromise. Further evaluation can include an upper gastrointestinal series, 24-hour pH probe monitoring, and radionuclide gastric emptying study. Initial therapeutic efforts should be medical: thickening of feedings, maintenance of upright position after feedings, reduction in use of theophylline for bronchodilation, and lowering of gastric acidity. If GER is contributing to worsening lung disease and medical therapy is unsuccessful, infants may benefit from fundoplication (24,25).
As infants with BPD grow, pulmonary symptoms (bronchospasm, chronic cough, shortness of breath) generally improve, and reliance on daily medications (low-flow oxygen, diuretics, bronchodilators) is reduced. Long-term sequelae of BPD, however, are seen in more than one-half of survivors. Follow-up of infants 20 years after premature birth (birthweights of more than 1,000 g) indicated that 76% of survivors showed abnormalities in pulmonary
function (26). In addition, among survivors, growth was reduced, neurologic sequelae were more frequent, and educational outcomes were significantly worse.
function (26). In addition, among survivors, growth was reduced, neurologic sequelae were more frequent, and educational outcomes were significantly worse.
Intracranial Hemorrhage
Risk of intracranial hemorrhage is directly associated with degree of prematurity. Severe brain injury with immediate (hemodynamic instability, seizures, rapid increase in anterior fontanelle pressure, anemia) and long-term [deafness, blindness, seizures, cerebral palsy (CP)] consequences is observed in 6% to 7% of very-low-birth-weight (VLBW) (less than 1,500 g) infants who survive intracranial hemorrhage. Prematurity, however, is not the sole determinant of prognosis. Several studies have documented a decrease in the incidence of intraventricular-periventricular hemorrhage (IV/PVH) during the past decade. Estimates suggest that 16% to 25% of very-low-birthweight infants sustain some degree of IV/PVH.
Two mechanisms account for the susceptibility of preterm infants to IV/PVH (27). First, these hemorrhages arise in the germinal matrix, with a rich vascular system that appears to be relatively fragile. The normal involution of this structure during the third trimester by about 34 weeks of gestation partially explains the decreasing risk of significant hemorrhage that accompanies increasing gestational age. Second, in contrast to rigorous autoregulation in adults, regulation of cerebral blood flow in premature infants is pressure passive. This dysfunctional autoregulation leads to greater pressure variations in the fragile vascular system of preterm infants. Both arterial and venous blood pressure can contribute to the pathogenesis of hemorrhage, particularly in instances of increased intrathoracic pressure associated with pneumothorax. Rapid changes in serum osmolality have also been implicated in the development of IV/PVH. These observations have led to more judicious use of rapid volume expansion in premature infants, slower administration of intravenous medications with high osmolality (e.g., sodium bicarbonate), and avoidance of rapid intravenous administration of solutions with glucose concentrations greater than 10%.
In light of the heterogeneous pathogenesis of IV/PVH, attempts at prevention have taken several routes (27). Prenatally, the most important interventions are prolongation of the healthy pregnant state and delivery at a site where optimal neonatal resuscitation efforts are available. Administration of antenatal steroids to accelerate fetal phospholipid pulmonary maturation has also been noted to reduce the postnatal risk of IV/PVH. Postnatally, access to rapid resuscitation and stabilization and avoidance of rapid changes in intrathoracic and intravascular pressure are important in preventing IV/PVH. Pharmacologic interventions aimed at stabilization of brain blood flow velocity (e.g., sedation or muscle relaxation) and prevention or correction of coagulation disturbances have also been attempted with varying degrees of success.
The prognosis after IV/PVH depends on initial severity, concomitant parenchymal injury, and development of posthemorrhagic hydrocephalus. Severity of injury is described by the extent of bleeding assessed by cranial ultrasound examination. When hemorrhage is limited to the area of the germinal matrix alone (grade I), no adverse immediate or long-term outcomes have been observed in careful clinical studies. More extensive hemorrhage into the lateral ventricles (grade II), from which the blood eventually is cleared, is associated with major neurologic sequelae (e.g., blindness, deafness, cerebral palsy, seizure disorder) in 10% to 15% of infants. Some infants with intraventricular hemorrhage develop varying degrees of ventricular enlargement (grade III), presumably secondary to reactive arachnoiditis as blood degradation products collect at the arachnoid granulations and impair cerebrospinal fluid reuptake. Rapid (within days), severe ventricular enlargement is associated with adverse outcome in about 40% of infants. In addition, large intraventricular hemorrhages may be accompanied by hemorrhagic infarctions in the surrounding periventricular white matter (grade IV). In contrast to the isolated germinal matrix hemorrhage or the intraventricular hemorrhage without significant ventriculomegaly, both extensive hydrocephalus and significant periventricular injury are associated with a more than 90% risk of major, long-term neurodevelopmental sequelae. About one-half of infants with ventriculomegaly associated with intraventricular hemorrhage require some treatment for relief of hydrocephalus. These interventions include serial lumbar punctures for 7 to 14 days, an external ventriculostomy or reservoir, or a ventriculoperitoneal or subgaleal shunt.
Neuroimaging and Central Nervous System Prognosis in Premature Infant
In light of these risks of injury and long-term sequelae, neuroimaging is used frequently because clinical evaluation of these high-risk infants may not provide adequate diagnostic or prognostic information. Not only can neuroimaging help with diagnosis of brain injury in the at-risk infant thereby assisting medical management, but also in detection of lesions that are associated with long-term neurodevelopmental disability. At the present time cranial ultrasonography (US) and magnetic resonance imaging (MRI) are the major imaging techniques most widely used to evaluate the premature neonate (28).
Cranial Ultrasound
This technique has been widely used and has the advantage of portability. In studies that compare neuropathological findings with ultrasound diagnoses, ultrasound findings
were 76% to 100% accurate for diagnosis of grades 1, 3, and 4 and to lesser extent grade 2 lesions of intraventricular hemorrhage (IVH) (28).
were 76% to 100% accurate for diagnosis of grades 1, 3, and 4 and to lesser extent grade 2 lesions of intraventricular hemorrhage (IVH) (28).
Nearly 25% of infants with a GA of less than 30 weeks have significant cranial US abnormalities (grades 3 and 4 IVH, cystic periventricular leukomalacia (PVL), and ventriculomegaly) that may impact acute and long-term care. Routine screening cranial US should be performed in this group.
Grades 3 and 4 IVH, cystic PVL, and moderate to severe ventriculomegaly detected by cranial ultrasound are significantly associated with CP at 2 to 9 years of age in VLBW premature infants (ten fold increased risk of adverse outcome). Grade 4 IVH and ventriculomegaly are significantly associated with mental retardation and neuropsychiatric disorders in childhood.
Magnetic Resonance Imaging
This technique is more sensitive than cranial US for detection of white matter lesions, hemorrhage, and PVL in the first week of life but usually requires transport of a critically ill infant to an MRI scanner (29).
Apnea of Prematurity
Apnea of prematurity (AOP) is the cessation of airflow, whether obstructive, central, or mixed, lasting 10 to 20 seconds that may be accompanied by cyanosis or bradycardia. The frequency of AOP increases with decreasing GA. Approximately 25% of infants weighing less than 2,500 g have one episode of apnea. The incidence increases to 90% in infants less than 1,000 g. The onset is often between 5 and 10 days of life.
AOP is usually of mixed etiology with both central and obstructive mechanisms playing a role. Pharyngeal competence in premature infants contributes significantly to AOP. During rapid eye movement sleep, the predominant form of premature sleep, breathing efforts are disorganized. The AOP episode may begin with decreased pharyngeal dilation followed by breathing efforts that lead to further airway collapse, then reflex swallowing and/or central apnea. Premature infants respond to hypoxia with an initial increase in minute ventilation, followed by a return to baseline or a decreased rather than an increased minute ventilation, as is seen in older infants and children. Premature infants also respond to hypercarbia by decreasing respiratory effort. Thus, the cycle of apnea can lead to a continuous spiral unless monitored and treated appropriately.
AOP is a diagnosis of exclusion. All infants at risk for AOP should have continuous monitoring of respiratory rate, heart rate, and pulse oximetry. Upon detection of an apneic event, evaluation must be individualized for each infant. When one or more apneic events represent a significant change in clinical course, complete physical examination should be the first intervention to identify evidence for signs of systemic bacterial or viral infection, necrotizing enterocolitis, PDA, intracranial hemorrhage, anemia, or electrolyte abnormalities. Initial workup may include a search for infectious etiology [complete blood cell count (CBC), blood culture, urinalysis and urine culture, nasopharyngeal swab for respiratory viruses, lumbar puncture, and chest X-ray]. Metabolic causes should also be excluded (e.g., hypoglycemia, hypothermia, hyponatremia, acidosis). Cardiac and neurologic (i.e., seizures) disorders should also be considered in the differential. Airway obstruction, whether by positioning or reflux of stomach contents, should be ruled out. Neck flexion may result in airway compromise. Placing the neck in a neutral to slightly extended position may improve airway caliber. GER may be present in premature infants, leading to a bolus of fluid to the oropharynx, which results in reflex-meditated closure of the glottis. An exaggerated reflex may result in a persistently closed glottis, despite removal of the obstruction. Prophylactic antibiotics may be started if signs or symptoms of infection are present, and any metabolic abnormalities should be corrected.
The goal of treatment for AOP is to decrease the number of episodes and prevent reintubation and mechanical ventilation. Most infants will outgrow AOP by 35 to 36 weeks postconceptual age. Those infants born at less than 30 weeks gestation may be started on pharmacotherapy presumptively (30). Those infants closer to 34 weeks corrected GA are often watched closely for spontaneous resolution of spells. Nonpharmacologic means include tactile stimulation and nasal continuous positive airway pressure (CPAP). Nasal CPAP appears to improve pharyngeal tone and decreases the frequency of apneic spells (31). A newer mode of nasal CPAP may improve results with this intervention. Nasal intermittent positive pressure ventilation has been studied in several small trials and appears to be more effective in reducing the number of apneas than CPAP alone (32). Gastric distension leading to cessation of feeds and possible gastric rupture was not seen in these studies. Larger-scale studies to determine the safety and efficacy of this newer therapy are underway.
Pharmacologic interventions include most commonly the methylxanthines (theophylline and caffeine). Their mechanism of action is not clearly understood. They appear to stimulate the respiratory center, leading to increased ventilation, reduced rapid eye movement sleep, improved skeletal muscle contraction, and increased cardiac output. Caffeine has a wider therapeutic window and safer side effect profile than theophylline. In general, very few side effects are seen with caffeine.
Cessation of pharmacotherapy usually occurs when the infant is free of events and approaching the 37 weeks postconceptional age. If apneic events persist past 37 weeks postconceptional age, further evaluation may be needed.
Infants with a history of AOP are watched in the hospital off methylxanthines for typically 7 days to allow for complete elimination of the drug. Home monitoring is typically not necessary for AOP, except in the rare instances that the infant is discharged home on pharmacotherapy, has a significant upper airway obstructive component, or has persistent apnea of infancy (33). Infants with a history of AOP are not at increased for sudden infant death syndrome (SIDS), although prematurity is an independent risk factor for SIDS.
Infants with a history of AOP are watched in the hospital off methylxanthines for typically 7 days to allow for complete elimination of the drug. Home monitoring is typically not necessary for AOP, except in the rare instances that the infant is discharged home on pharmacotherapy, has a significant upper airway obstructive component, or has persistent apnea of infancy (33). Infants with a history of AOP are not at increased for sudden infant death syndrome (SIDS), although prematurity is an independent risk factor for SIDS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree