Clinical Principles of Abdominal Surgery
George W. Holcomb III
Daniel J. Ostlie
Children’s Mercy Hospital, University of Missouri at Kansas City School of Medicine, Kansas City, Missouri 64108.
Children’s Mercy Hospital, University of Missouri at Kansas City School of Medicine, Kansas City, Missouri 64108.
PERITONEUM
The peritoneal cavity is divided into two separate cavities connected by the epiploic foramen. This opening connects the lesser sac behind the stomach and liver with the greater peritoneal cavity. Anatomically, the general peritoneal sac is divided into several spaces, owing to the fixed visceral attachments to the retroperitoneum. The right and left subphrenic spaces are common locations for abscess formation. The left and right subhepatic spaces are located between the liver and transverse mesocolon, and are separated by the epiploic foramen. The right subhepatic space is a common site for inflammatory processes arising from the biliary system, the head of the pancreas, and the duodenum. Located to the right of the small bowel mesentery, the right infracolic space has direct communication with the right subhepatic space. Viscera included in this space are the appendix, cecum, and right female adnexa. Because of its location, inflammatory processes from the right infracolic region may spread to the lesser sac. Similarly, the left infracolic space is located left of the obliquely oriented small bowel mesentery and contains the sigmoid colon and left female adnexa. The remaining spaces include the pelvis and lateral paracolic gutters. Inflammatory processes tend to remain localized to these spaces if host-defense mechanisms are effective.
The peritoneum is the serous membrane that encloses the abdominal cavity and is reflected onto its associated viscera. This monolayer lining consists of mesothelial cells in a continuous layer resting on a basement membrane constructed from a collagen lattice. The apical surfaces of the mesothelial cells are lined with microvilli so the entire surface area of the peritoneum is approximately equal to that of the skin.
A complex circulation system present within the peritoneum provides a small volume of fluid for lubricating purposes that promotes mobility between visceral organs. Excess fluid accumulates in the subdiaphragmatic spaces and is actively pumped into the thoracic duct with diaphragmatic contraction. Although clearance of excess peritoneal fluid is effective, this process may also promote entry of bacteria into the systemic circulation, causing bacteremia. An additional fluid transfer mechanism is the lymphatic system. The diaphragmatic and peritoneal lymphatics are large and do not have valves or occlusive junctions. With certain diseases and during peritoneal dialysis, these lymphatics play an important part in transport of fluids. In addition to clearance through the previously mentioned transport mechanisms, the peritoneum contains resident macrophages, mast cells, basophils, and eosinophils, which respond to inflammation and infection.
In response to injury, peritoneal healing differs from skin. The entire peritonea defect becomes endothelialized simultaneously, not from the borders, as occurs with epidermalization of skin wounds (1,2). Moreover, the granulation and contraction that occurs around the edges of
skin wounds does not occur during peritoneal healing. Reepithelialization of the parietal peritoneum occurs within 5 to 6 days of injury with complete repair by 8 days. Healing of the visceral peritoneum does not differ significantly from that of the parietal peritoneum, but it may occur 1 to 2 days earlier. Several studies have confirmed that reepithelialization from peritoneal injury appears to occur faster in immature versus mature rats.
skin wounds does not occur during peritoneal healing. Reepithelialization of the parietal peritoneum occurs within 5 to 6 days of injury with complete repair by 8 days. Healing of the visceral peritoneum does not differ significantly from that of the parietal peritoneum, but it may occur 1 to 2 days earlier. Several studies have confirmed that reepithelialization from peritoneal injury appears to occur faster in immature versus mature rats.
Innervation
The parietal peritoneum is derived from the somatopleural mesoderm, whereas the splanchnopleural mesoderm gives rise to the visceral peritoneum. Because of this different derivation, the parietal peritoneum shares its neurovascular and lymphatic connections with the musculoskeletal abdominal wall, and the visceral peritoneum shares its connections with its associated visceral organs. For this reason, perception of painful stimuli is markedly different between the visceral and parietal peritoneum.
Visceral pain is perceived through neuronal pathways from the lower thoracic and lumbar splanchnic nerves and from the parasympathetic pathways of the vagus and sacral plexus. As such, visceral pain is usually dull and aching, and may be poorly localized. A steady, vague pain usually occurs after inflammation of the visceral peritoneum, whereas severe, intermittent, colicky pain results from hollow visceral obstruction. Moreover, nausea, vomiting, and sweating commonly occur with visceral inflammation.
Generalized discomfort in the epigastric, periumbilical, and hypogastric regions, respectively, may correspond with foregut, midgut, and hindgut inflammation. Inflammatory processes in the stomach, pancreas, duodenum, and biliary system may initially be manifested with vague epigastric discomfort. Similarly, small bowel and right and transverse colon inflammation is sensed as periumbilical discomfort, whereas left colon, sigmoid, and rectal pain is perceived as lower abdominal pain.
Because neuronal innervation to the parietal peritoneum is derived from the somatic nerves supplying the adjacent abdominal wall structures and skin, inflammatory stimulation is usually more localized, intense, and constant than with visceral pain. Stimulation of the parietal peritoneum is usually responsible for localization of the disease process. An example of the difference between recognition of visceral and parietal stimuli is found with appendicitis. Early appendiceal distention may be manifested by a periumbilical dull or cramping discomfort. With inflammation of the overlying parietal peritoneum, localization of the pain to the right lower quadrant typically develops, leading to the diagnosis of appendicitis.
Adhesions
Peritoneal adhesions occur as a by-product of laparotomy and inflammation. A number of pharmacologic agents, including corticosteroids, nonsteroidal antiinflammatory agents, and dextran have been used to decrease postoperative adhesions. Although corticosteroids have not been found to be useful, some benefit is suggested for ibuprofen and dextran (3,4). Barrier agents, such as polytetrafluoroethylene and oxidized regenerated cellulose, have been used in gynecologic studies (5,6,7). With intact hemostasis, these agents have been observed to be effective in localized peritoneal injury. Instillation of crystalloid solution has also been tried in an effort to decrease postoperative adhesions, but this isolated agent has not been effective (4,8).
Controversy exists as to whether peritoneal closure to cover areas denuded by previous dissection is beneficial. Animal studies demonstrate enhanced adhesion formation to suture lines when the peritoneum is closed over denuded tissue (9). Experimental evidence indicates that areas stripped of peritoneum heal satisfactorily. Moreover, suturing of the peritoneum may actually increase the formation of adhesions (10,11). It has been postulated that one way to reduce postoperative adhesions is through the use of laparoscopic surgery. In a prospective multicenter trial, second-look laparoscopy and adhesiolysis were performed (12). Of the areas where laparoscopic adhesiolysis had been previously performed, 67% contained adhesions at second-look laparoscopy. De novo adhesion formation, however, was substantially reduced by the laparoscopic surgery—new adhesions were noted in only 16% of the patients. Reduced adhesions were also found by Garrard and colleagues (13) in an experimental study comparing laparotomy with laparoscopy. Therefore, it appears that laparoscopic surgical techniques may reduce de novo adhesion formation, although reformation of old adhesions that have been lysed continues to be a major problem.
ACUTE ABDOMINAL CONDITIONS
Acute abdominal diseases in infants, children, and adolescents are often different from those conditions found in adults. Congenital abnormalities are usually the cause for abdominal symptoms in infants and young children. As the child grows older, diseases and symptoms common to adults become more prevalent.
The approach to the patient depends largely on the patient’s age. Historical data may be lacking in infants and children too young to verbalize their complaints specifically. Often, young parents are not as perceptive as grandparents in detailing an accurate history of the young child’s symptoms. Moreover, children may be placed in day-care centers, and a detailed history may actually be unknown to the parents.
Physical examination is key to determining the cause of a child’s illness. The young child may be frightened and apprehensive, thus precluding an accurate examination. It may be helpful to examine an infant or young child in
the parent’s lap where they feel secure and comfortable, allowing a more accurate evaluation. In addition, abdominal palpation may be easier to perform and more information may be gained with the use of a stethoscope rather than the physician’s hand. Often, children relax their abdominal muscles if they believe the physician is merely trying to listen to abdominal sounds rather than attempting to elicit tenderness. It is usually best to examine the region of suspected abdominal pathology last to get a more accurate interpretation of the abdominal examination. When examining the location of symptoms first, the child may become apprehensive and sense that the remainder of the examination will also be painful. When examining young children and adolescents, another helpful maneuver is to distract the child from the examination through talking. Questions about school, play, siblings, family, and so forth may distract the child from the palpation and permit a more accurate evaluation.
the parent’s lap where they feel secure and comfortable, allowing a more accurate evaluation. In addition, abdominal palpation may be easier to perform and more information may be gained with the use of a stethoscope rather than the physician’s hand. Often, children relax their abdominal muscles if they believe the physician is merely trying to listen to abdominal sounds rather than attempting to elicit tenderness. It is usually best to examine the region of suspected abdominal pathology last to get a more accurate interpretation of the abdominal examination. When examining the location of symptoms first, the child may become apprehensive and sense that the remainder of the examination will also be painful. When examining young children and adolescents, another helpful maneuver is to distract the child from the examination through talking. Questions about school, play, siblings, family, and so forth may distract the child from the palpation and permit a more accurate evaluation.
The rectal and pelvic examination in infants and children may not be as helpful as in adults. Rectal examination is not necessary in every case of abdominal discomfort. When indicated, however, it may be useful in eliciting a cause for the symptoms. Similarly, rarely do prepubescent girls need a pelvic examination. Rectal examination may serve the same purpose by documenting the presence of a cervix, uterus, or pelvic abscess and other masses. External genitalia examination, however, is an important component to evaluation of the child with abdominal symptoms because vaginal atresia, imperforate hymen, or foreign bodies can cause abdominal symptoms.
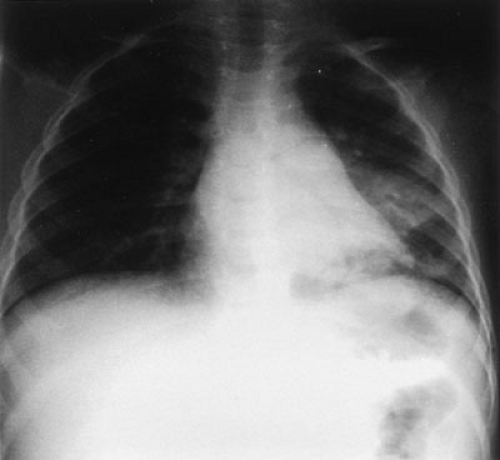
Laboratory data can be useful in children with abdominal symptoms. Hematocrit, leukocyte count, and urinalysis are helpful in most children with relevant complaints. Other, more specific tests, such as serum amylase, sedimentation rate, liver function tests, and clotting tests, are reserved for investigation of suspected organ cause. Sometimes, radiographic tests are the most helpful in evaluating abdominal symptoms in infants and children. These may be especially advantageous in infants who present with abdominal distention, nausea, and vomiting. In general, supine and upright radiographs should be performed when plain films are requested. In infants, a left decubitus radiograph serves the same purpose as an upright and may demonstrate evidence of pneumoperitoneum and obstruction. A prone cross-table lateral radiograph is helpful in documenting the presence or absence of air in the rectum. The absence of rectal gas may be useful in diagnosing conditions, such as small bowel obstruction, intestinal atresia, and meconium ileus. Chest radiographs may also be helpful because pneumonia can cause upper abdominal discomfort, particularly with pleurisy. When evaluating a child with fever and abdominal complaints, it is important to realize that pneumonia may not be apparent if the child is dehydrated, but will be evident on subsequent chest radiographs after rehydration (Fig. 67-1). Ultrasound examination can also be useful in evaluating abdominal symptoms. Most pediatric radiologists are adept at imaging both solid and hollow intestinal viscera and frequently can determine the cause of a child’s complaints. It is important, however, to provide the ultrasonographer with as much information as possible relating to the history and examination so the study is directed toward the suspected organ. Computed tomography (CT) examination may also be employed in cases in which plain radiographs and sonographic studies have failed to determine the diagnosis. Moreover, CT and even magnetic resonance imaging may be required when a neoplasm is suspected. Specific abdominal conditions are addressed in individual chapters.
SURGICAL TECHNIQUES
Diagnostic Laparoscopy
Although Stephen Gans (14) described laparoscopy for several pediatric uses in the 1970s, few pediatric surgeons incorporated this into their practices prior to the late 1980s. Most laparoscopic procedures in adults were performed by gynecologists. After the sentinel report of laparoscopic cholecystectomy by Reddick and Olsen in 1989 (15), a revolution in endoscopic surgery began. Now, almost every open procedure in an adult has an endoscopic counterpart. Application of this endoscopic approach was less rapid in children, however, primarily because the apparent advantages, such as decreased hospitalization, reduced discomfort, improved cosmesis, and a faster return to work, were
not believed to be as important to children by many pediatric surgeons. Moreover, small incisions are routinely employed for a variety of pediatric surgical procedures, and use of several small incisions for endoscopic surgery was not obviously beneficial. However, during the late 1990s and subsequent, the use of laparoscopy and thoracoscopy by pediatric surgeons has increased dramatically and these endoscopic approaches are now considered routine rather than revolutionary.
not believed to be as important to children by many pediatric surgeons. Moreover, small incisions are routinely employed for a variety of pediatric surgical procedures, and use of several small incisions for endoscopic surgery was not obviously beneficial. However, during the late 1990s and subsequent, the use of laparoscopy and thoracoscopy by pediatric surgeons has increased dramatically and these endoscopic approaches are now considered routine rather than revolutionary.
Although the principles of laparoscopic surgery for children are similar to those in adults, several unique differences require special attention. Because of the smaller abdominal cavity in children, especially infants, it is important to separate the cannulas as widely as possible to provide adequate working space. Placement of the ports too closely hinders the operation. As an example, for cholecystectomy in adults, the epigastric cannula is usually positioned in the midline of the epigastrium, and the right lower port is often placed just below or just above the level of the umbilicus. In a young child, however, the epigastric incision should be situated more to the patient’s left, and the right lower port may be placed in the inguinal crease to allow sufficient separation.
Infants and young children have pliable abdominal walls. Therefore, when introducing cannulas with sharp trocars, it is critical to advance the trocar cautiously as it penetrates the peritoneum in order to prevent injury to the underlying viscera and intestine. Once the sharp stylet has penetrated the peritoneum, it is prudent to direct it anteriorly above the underlying intestine, viscera, and major vessels as the trocar and cannula are inserted deeper into the abdominal cavity. In addition, use of the Veress needle in children and, in particular, infants, is discouraged for creation of pneumoperitoneum. A much safer technique is an umbilical cutdown with insertion of the umbilical cannula into the peritoneal cavity under direct vision. This cutdown technique should prevent serious injury related to a blind puncture of the peritoneal cavity with the Veress needle.
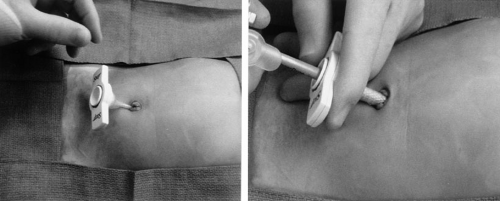
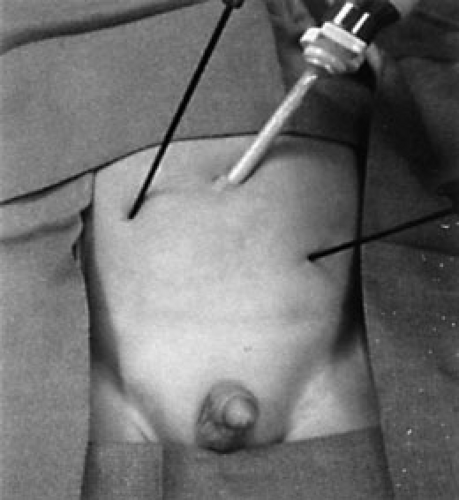
Because of the previous concerns with the pliable abdominal wall in infants and young children, many pediatric surgeons prefer using the StepTM technique (US Surgical, Norwalk, CT). When introducing the initial umbilical cannula, we prefer to insert the expandable sheath (without the Veress needle) into the abdominal cavity followed by the cannula with the blunt trocar (Fig. 67-2). If an accessory cannula is used, then the Veress needle and expandable sheath are inserted into the abdominal cavity under telescopic visualization. The Veress needle is removed, and the cannula with blunt trocar is inserted through the expandable sheath. In this way, a sharp stylet is not used and potential injury to underlying viscera is minimized. Currently, these cannula sheaths are available as disposable units or reusable instruments. With the reusable instrument, the disposable portion is the cap, sheath, and Veress needle, but the blunt stylet and shaft of the cannula are reusable. Despite the resuable nature, there is added cost to using the disposable portion of these cannulas. Because of this added cost, several years ago, we began to insert the instruments directly through the abdominal wall without the use of a cannula, when possible. Between November 1999 and March 2003, we used minimal access stab incisions, rather than cannulas, for patients undergoing foregut, biliary, adrenal, splenic, colonic, and genitourinary operations. A single cannula was used for telescope access and, in select cases, a second cannula was needed for unique instruments, such as endoscopic staplers or ultrasonic shears. The abdominal wall stab incisions were used in the remaining patients (Fig. 67-3). During this time
period, 511 minimal access procedures were performed and pneumoperitoneum was maintained in all cases. In 308 of these cases, a single cannula was used and a second cannula was placed in 203 patients. In total, 1,337 reusable cannula systems (Veress needle, expandable sheath and cap) were saved using this minimal access technique. At a charge to the patient of $140 per cannula system, overall cost savings were $187,180 (16).
period, 511 minimal access procedures were performed and pneumoperitoneum was maintained in all cases. In 308 of these cases, a single cannula was used and a second cannula was placed in 203 patients. In total, 1,337 reusable cannula systems (Veress needle, expandable sheath and cap) were saved using this minimal access technique. At a charge to the patient of $140 per cannula system, overall cost savings were $187,180 (16).
Another special concern in children is excessive abdominal insufflation. In a research model, Liem and associates (17) demonstrated a direct correlation between insufflation pressure using CO2 and hypercapnea. In addition, there was marked acidemia, hypoxia, and increased exhaled CO2 with higher insufflation pressures. The authors recommended using insufflation pressures of less than 15 mm Hg. Many pediatric surgeons, however, continue to use 15 mm Hg as the maximum inflating pressure without apparent adverse clinical sequelae.
Absolute contraindications to laparoscopy include abdominal wall sepsis at the cannula site, an uncorrectable bleeding disorder and the inability to create a pneumoperitoneum. A common scenario arises when an infant with severe lung disease, such as respiratory distress syndrome and/or an oxygen requirement, requires a fundoplication. Depending on the patient’s condition, it may not be possible to establish a pneumoperitoneum without compromising oxygenation or ventilation. Whether a laparoscopic operation is possible depends on the individual patient’s condition. A preoperative discussion with the anesthesiologist is beneficial before scheduling the laparoscopic operation.
Diagnostic Laparoscopy
Laparoscopy can be employed for diagnostic purposes or for performance of a definitive operation. Diagnostic laparoscopy can be valuable in infants and children. Indications for diagnostic laparoscopy include evaluation of a nonpalpable testis, determination of the presence or absence of a contralateral patent processus vaginalis (CPPV) in a child with a known unilateral inguinal hernia, evaluation for chronic abdominal pain, diagnostic assessment for the presence of appendicitis, evaluation of traumatic injury, and in children with cancer.
A thorough preoperative conference is arranged with the parents and child, if age appropriate, at which time the procedure, risks, and benefits are discussed. General endotracheal anesthesia is usually preferred, but mask anesthesia is possible for short cases (18). An orogastric tube is inserted for gastric decompression, and the bladder is emptied using a Credé maneuver. The bladder is generally not catheterized, especially in young boys, to avoid iatrogenic urethral injury.
The abdomen is prepped and draped widely. As previously mentioned, an umbilical cutdown is performed for initial access to the abdominal cavity. The expandable sheath is introduced into the abdominal cavity followed by insertion of the cannula with a blunt trocar through the sheath. Use of the Veress needle for initial insufflation is discouraged, owing to the risk of injury to the abdominal viscera and vasculature. In general, a 5-mm cannula and telescope are used for most diagnostic procedures. The incision is well hidden in the umbilicus, and the visualization is better than with a 3-mm telescope. Moreover, the 5-mm umbilical fascial defect is easier to close than the smaller 3-mm opening. When operative laparoscopy is required, additional ports can be inserted as needed. Insufflation pressures up to 15 mm Hg have not caused significant adverse clinical sequelae in the authors’ experience with more than 1,000 laparoscopic operations.
Evaluation for a Nonpalpable Testis
The use of laparoscopy in boys with nonpalpable testes has a number of advantages and few disadvantages. Some surgeons who do not favor this approach argue that a complete examination of the inguinal region and abdominal cavity can be performed through an inguinal approach and that it is rarely necessary to resort to a two-stage procedure for orchiopexy (19). Proponents of the laparoscopic approach emphasize two valid points. First, some intraabdominal testes are not identified at the time of inguinal exploration and are missed even by experienced surgeons (20,21). Second, a laparoscopic approach allows the surgeon to defer the second-stage Fowler-Stephens orchiopexy, when indicated, preventing disruption of the vasal collateral vessels that might occur with extensive dissection using an initial inguinal incision. With a two-stage approach for the abdominal testis (initial laparoscopy with
vascular ligation followed later by Fowler-Stephens orchiopexy), the success rate should be greater than a one-stage approach, although the reported data are at present insufficient (22,23).
vascular ligation followed later by Fowler-Stephens orchiopexy), the success rate should be greater than a one-stage approach, although the reported data are at present insufficient (22,23).
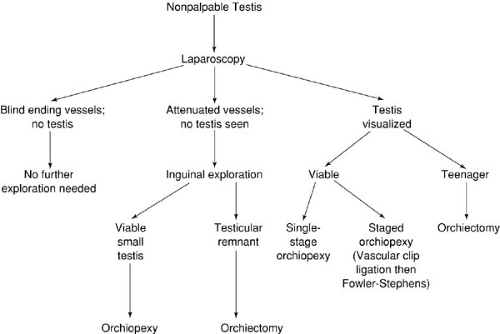
Under general anesthesia before laparoscopy, a final examination is performed to ensure the testicle is not palpable. When the testis is located in the inguinal canal or scrotum with the patient under anesthesia, then laparoscopy is not necessary. When the testis is not palpated, however, laparoscopy is performed, and the findings help the surgeon determine whether vessel ligation, orchiectomy, or an inguinal exploration is indicated (Fig. 67-4).
At diagnostic laparoscopy for the nonpalpable testis, several findings may be apparent. If a testis is found intraabdominally and the vascular leash appears short, then initial endoscopic vessel ligation can be performed. Great care should be taken to ensure the ureter is not injured. Six to 9 months later, the patient returns for a Fowler-Stephens orchiopexy, with the testicle then being nourished by collateral vessels surrounding the vas deferens. At the initial diagnostic laparoscopy, if there is no evidence of a testis, but a normal vascular pedicle and vas deferens are seen entering a closed internal ring, then inguinal exploration is indicated. Although the vas deferens and testicular vessels are likely to lead the surgeon to an atrophic testicular remnant, Turek and colleagues (24) found 6% of such specimens to have seminiferous tubules with germal elements. They recommended excision of these testicular remnants because of increased malignant potential. On occasion, however, a small, but viable, testis is found, and orchiopexy is possible. If the testis is identified to be high in the inguinal canal, but not truly intraabdominal (so-called peeping testis), then a one-stage orchiopexy may be possible. At laparoscopy, if the testicular vessels are noted to end blindly and do not enter the internal ring, then inguinal exploration is not indicated. Finally, in teenagers with intraabdominal testes or in younger children with atrophic intraabdominal testes, orchiectomy can be performed laparoscopically or through a separate inguinal incision.
Laparoscopy for Contralateral Patent Processus Vaginalis (CPPV)
A controversial subject among pediatric surgeons continues to be whether to perform a contralateral exploration under the same anesthesia in an infant or child with a known unilateral inguinal hernia. Advocates of routine bilateral exploration note that between 40% and 60% of children have a CPPV, and bilateral exploration prevents the need for a repeat operation with hernia repair in the future. A second anesthesia and the cost of a second procedure are avoided using this approach (25,26). Proponents for repair of the unilateral hernia alone argue that only 10% to 30% of children undergoing unilateral hernia repair return with a symptomatic contralateral hernia; therefore, in most children, an unnecessary contralateral operation would be avoided (27,28). Moreover, there is a small risk of injury to the testicle and vas deferens during inguinal exploration, which would also be avoided. One report of 313 children undergoing inguinal herniorrhaphy documented segments of vas deferens in five specimens (1.6%) (25). In another review, 160 infants with unilateral or bilateral repair were followed for up to 20 years and were discovered to have a 2% incidence of testicular atrophy (27).
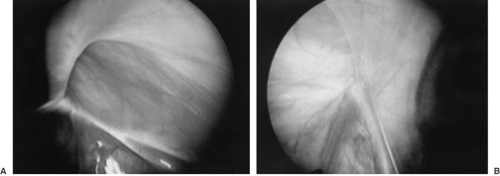
With the increased use of diagnostic laparoscopy, a study was undertaken to ascertain whether laparoscopy would be beneficial in the evaluation of a CPPV in a child with a known unilateral inguinal hernia (29). After
induction of general endotracheal anesthesia, the patient was examined and the surgeon asked to document if a CPPV was present on clinical examination under anesthesia (EUA). Diagnostic peritoneoscopy was then performed. Pneumoperitoneum was created to a pressure of 15 mm Hg, and the inguinal canal and scrotum were inspected to see if insufflation alone would be sufficient for the diagnosis of CPPV. Diagnostic peritoneoscopy was then performed (Fig. 67-5). These patients (1,432 of them) younger than 10 years of age were evaluated and laparoscopy was possible in 94.9% of them. In 548 patients (38%), a CPPV was identified. At EUA, it was predicted that 427 of these 1,359 patients would have a CPPV. The presence of a CPPV was confirmed in 188 (44%). Also, it was believed that 932 would not have a CPPV on EUA, but a CPPV was seen in 361 (39%). Thus, it is concluded that physical examination, even under anesthesia, is a poor prediction of a CPPV. In addition, a bulge was seen or crepitus palpated on the contralateral side with creation of the pneumoperitoneum in less than 10% of patients found to have a CPPV on laparoscopy. Therefore, laparoscopy should be considered the gold standard for evaluating for a CPPV (30). However, in no way does laparoscopy select who will have a symptomatic CPPV in the future.
induction of general endotracheal anesthesia, the patient was examined and the surgeon asked to document if a CPPV was present on clinical examination under anesthesia (EUA). Diagnostic peritoneoscopy was then performed. Pneumoperitoneum was created to a pressure of 15 mm Hg, and the inguinal canal and scrotum were inspected to see if insufflation alone would be sufficient for the diagnosis of CPPV. Diagnostic peritoneoscopy was then performed (Fig. 67-5). These patients (1,432 of them) younger than 10 years of age were evaluated and laparoscopy was possible in 94.9% of them. In 548 patients (38%), a CPPV was identified. At EUA, it was predicted that 427 of these 1,359 patients would have a CPPV. The presence of a CPPV was confirmed in 188 (44%). Also, it was believed that 932 would not have a CPPV on EUA, but a CPPV was seen in 361 (39%). Thus, it is concluded that physical examination, even under anesthesia, is a poor prediction of a CPPV. In addition, a bulge was seen or crepitus palpated on the contralateral side with creation of the pneumoperitoneum in less than 10% of patients found to have a CPPV on laparoscopy. Therefore, laparoscopy should be considered the gold standard for evaluating for a CPPV (30). However, in no way does laparoscopy select who will have a symptomatic CPPV in the future.
Other Indications
Evaluation for chronic abdominal pain is another valid indication for diagnostic laparoscopy in children. Despite multiple radiographic studies, laboratory tests, and physical examinations, an open laparotomy may be necessary in selected circumstances when abdominal pain persists or recurs. Laparoscopy has been very helpful in this circumstance (31). Telander reported an incidence of positive findings in 60% of 40 cases using laparoscopy for evaluation of chronic abdominal pain (Telander W.H., 1999 personal communication). Moreover, 30% of these 40 patients had an abnormal appendix on pathologic examination.
Diagnostic laparoscopy may also be useful in children with suspected appendicitis. In addition, this technique can be used to direct the best location for an open incision when the surgeon, for any reason, is not comfortable with continuation of the laparoscopic approach.
In addition to the previously mentioned indications, diagnostic laparoscopy may also be useful in other selected circumstances. Infants with ambiguous genitalia are good candidates for diagnostic laparoscopy for determination of their internal anatomy (32). It can be used in the evaluation of traumatic injuries in children in whom the radiographic and other noninvasive evaluations are equivocal. This is especially applicable to penetrating trauma in which the surgeon does not believe that the traumatic event, whether a low-velocity gunshot or stab injury, penetrated the abdominal cavity. Moreover, it does allow the surgeon to evaluate for internal injuries if the peritoneum was violated. Finally, diagnostic laparoscopy can be used in selected circumstances in children with cancer, especially when the radiographic evaluations are not diagnostic. It can be useful for determining resectability of a large lesion, such as a hepatoblastoma or neuroblastoma, followed by open exploration and resection, if appropriate. Second-look laparoscopy can be useful following adjuvant therapy in certain circumstances (Fig. 67-6). In a review several years ago from the Children’s Cancer Group, 24 children were documented to have undergone laparoscopy as part of their surgical management (33). Indications include evaluation for possible metastatic tumor or recurrent disease, consideration of a new mass for suspected cancer, and evaluation of hepatoblastoma for resectability.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree