EPIDEMIOLOGY
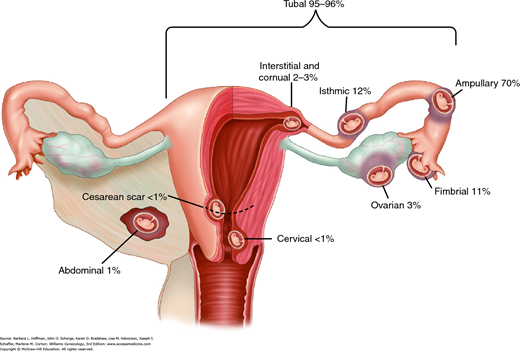
An ectopic or extrauterine pregnancy is one in which the blastocyst implants anywhere other than the endometrial lining of the uterine cavity. Nearly 95 percent of ectopic pregnancies implant in the fallopian tube. Other sites are shown in Figure 7-1, which reflects data from 1800 surgically treated ectopic pregnancies (Bouyer, 2002). Bilateral ectopic pregnancies are rare, and their estimated prevalence is 1 of every 200,000 pregnancies (al-Awwad, 1999).
Reported incidences rates of ectopic pregnancy are less reliable than in the past as outpatient treatment protocols render national hospital discharge statistics invalid. One estimate by Kaiser Permanente of North California was 2.07 percent of total pregnancies from 1997 to 2000 (Van Den Eeden, 2005). Hoover and colleagues (2010) queried a large claims database of privately insured women between 2002 and 2007 and calculated a rate of 0.64 percent. However, this may not accurately reflect the cases in higher-risk, lower-socioeconomic, uninsured populations. Stulberg and coworkers (2014) reviewed 2004 to 2008 Medicaid claims data from 14 states. They reported a rate of 1.4 percent and noted that black women were 46 percent more likely to experience an ectopic pregnancy than whites in this government-insured group.
Among several factors that help explain the incidence of ectopic pregnancies are: (1) greater sexually transmitted disease prevalence, (2) diagnostic tools with improved sensitivity, (3) tubal factor infertility, (4) delayed childbearing and accompanied use of assisted reproductive technology, and (5) increased intrauterine device (IUD) use and tubal sterilization, which predispose to ectopic pregnancy if the method fails (Ankum, 1996; Li, 2014a; Ljubin-Sternak, 2014).
Ectopic pregnancy remains the leading cause of early pregnancy-related death. Still, current diagnostic and treatment protocols have resulted in substantial declines in fatality rates. One analysis showed a 56-percent decline in the ectopic pregnancy mortality ratio between the 1980 to 1984 epoch and the 2003 to 2007 epoch. During this later span, African-American women were approximately three times more likely to die as a result of ectopic pregnancy complications than whites (Creanga, 2011). Inadequate access to gynecologic and prenatal care may partially explain this trend.
In most of these cases, death is directly related to severe hemorrhage from tubal rupture. Risk factors that increase the likelihood of tubal rupture include ovulation induction, serum β-human chorionic gonadotropin (β-hCG) level >10,000 IU/L, and never having used contraception (Job-Spira, 1999). Appreciation of these characteristics can aid prompt surgical intervention.
RISK FACTORS
Several risks have been linked with ectopic pregnancy (Table 7-1). Among these, documented tubal pathology, surgery to restore tubal patency, or tubal sterilization can all lead to obstruction and subsequent ectopic pregnancy. A woman with two prior ectopic pregnancies has a 10- to 16-fold increased chance for another (Barnhart, 2006; Skjeldestad, 1998).
| Factor | Odds Ratio (95% CI) |
| Prior ectopic pregnancy | 12.5 (7.5, 20.9) |
| Prior tubal surgery | 4.0 (2.6, 6.1) |
| Smoking >20 cigarettes per day | 3.5 (1.4, 8.6) |
| PID confirmed by laparoscopy or positive test for Chlamydia trachomatis | 3.4 (2.4, 5.0) |
| ≥3 prior spontaneous miscarriages | 3.0 (1.3, 6.9) |
| Age ≥40 years | 2.9 (1.4, 6.1) |
| Prior medical or surgical abortion | 2.8 (1.1, 7.2) |
| Infertility >1 year | 2.6 (1.6, 4.2) |
| Lifelong sexual partners >5 | 1.6 (1.2, 2.1) |
| Prior IUD use | 1.3 (1.0, 1.8) |
Smoking, which may be a surrogate marker for sexually transmitted infections, increases the ectopic pregnancy risk three- to fourfold in women who smoke more than one pack of cigarettes daily (Saraiya, 1998). The increased risk of ectopic pregnancy among smokers undergoing assisted reproductive technology was verified in a metaanalysis by Waylen and associates (2009). In addition, animal studies show that smoking alters oocyte cumulus complex pick-up and embryo transport through its effects on ciliary function and smooth-muscle contraction (Shaw, 2010; Talbot, 2005).
Assisted reproductive technology (ART) for sub- or infertile couples has a 0.8-percent incidence of ectopic pregnancy per transfer and 2.2 percent per clinical pregnancy (Coste, 2000). Interestingly, recent series note significant reductions in ectopic pregnancy rates at the time of in vitro fertilization (IVF) if frozen-thawed embryos (2.2 percent rate) are used rather than those from fresh cycles (4.6 percent) (Fang, 2015; Huang, 2014). In women undergoing IVF, the main risk factors for ectopic pregnancy are tubal factor infertility and hydrosalpinges (Strandell, 1999; Van Voorhis, 2006). Moreover, “atypical” implantation—that is, interstitial, abdominal, cervical, ovarian, or heterotopic—is more common following ART procedures. As a review, heterotopic pregnancy is an intrauterine pregnancy coexistent with an extrauterine pregnancy.
Older reproductive-aged women, specifically women aged 35 to 44 years, carry a threefold risk of ectopic pregnancy compared with those aged 15 to 25 years (Goldner, 1993). These have been attributed to age-related hormonal changes that alter tubal function (Coste, 2000).
Contraception lowers overall pregnancy rates and thereby lowers ectopic pregnancy rates. However, if pregnancy does occur, some methods increase the relative incidence of ectopic pregnancy. Examples include the levonorgestrel-releasing intrauterine system (Mirena) and copper IUD (ParaGard). In one study of 61,448 IUD users, 118 contraceptive failures were reported, and 21 of these were ectopic (Heinemann, 2015). Progestin-only contraceptive pills also pose a slightly increased risk because of their effects to diminish tubal motility. With tubal sterilization failure, ectopic pregnancy is a concern. In one study, this risk was 3.5 times greater in women younger than 28 years at the time of sterilization. This may be in part because of age-related fecundity. Of methods, higher ectopic rates were noted with laparoscopic partial salpingectomy and electrodestruction methods (Malacova, 2014).
PATHOPHYSIOLOGY
Acute inflammation has been implicated in the tubal damage that predisposes to ectopic pregnancies. Chronic salpingitis and salpingitis isthmica nodosa also contribute (Kutluay, 1994).
Of suspected agents, recurrent chlamydial infection causes intraluminal inflammation, subsequent fibrin deposition, and tubal scarring (Hillis, 1997). Moreover, persistent chlamydial antigens can trigger a delayed hypersensitivity reaction that promotes continued scarring despite negative culture results (Toth, 2000). Whereas endotoxin-producing Neisseria gonorrhoeae causes virulent pelvic inflammation that has a rapid clinical onset, the chlamydial inflammatory response is chronic and peaks at 7 to 14 days.
Inflammation within the fallopian tube can also arrest embryo progress and provide a premature proimplantation signal (Shaw, 2010). Specifically, oviduct interstitial cells of Cajal are specialized pacemaker cells responsible for oviduct motility and egg transport. Infections in mice by Chlamydia muridarum, which is similar to human Chlamydia trachomatis, lead to absent spontaneous pacemaker activity and may offer another explanation of how chlamydial infection increases ectopic pregnancy rates in humans (Dixon, 2009).
Another factor involved with oviductal transport of embryos is the cannabinoid receptor (CB1), which is mediated by endocannabinoid signaling. Chronic exposure to nicotine can affect endocannabinoid levels and lead to fallopian tube dysfunction (Horne, 2008).
The mechanism for ectopic pregnancy in women using ART has been a conundrum because the fallopian tube is typically bypassed. Revel and colleagues (2008) sought to establish the relationship between E-cadherin, an adhesion molecule, and tubal ectopic pregnancy implantation sites. They found E-cadherin strongly localized to the tubal embryo implantation site only in women who underwent IVF. This suggests a biologic rather than mechanical factor accounting for the ectopic pregnancies associated with IVF.
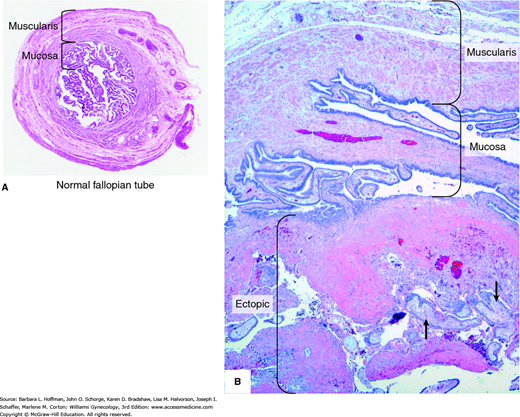
Once normal tubal transport has been disrupted, fallopian tube anatomy plays an important role in tubal pregnancy genesis. Namely, the fallopian tube lacks a submucosal layer beneath its epithelium. Therefore, a fertilized ovum can easily burrow through the epithelium and implant within tube’s muscularis layer (Fig. 7-2). As rapidly proliferating trophoblasts erode the muscularis layer, maternal blood pours into the spaces within the trophoblastic or the adjacent tissue.
With this process, the location of a tubal pregnancy may predict the extent of damage. Senterman and associates (1988) studied histologic samples from 84 isthmic and ampullary pregnancies. They reported that half of the ampullary pregnancies were intraluminal, and the muscularis was preserved in 85 percent of these. Conversely, isthmic gestations were found both intra- and extraluminally with greater disruption of the tubal wall. The timing of tubal rupture is also partially dependent on pregnancy location. As a rule, fallopian tubes rupture earlier if implantation is in the isthmic or ampullary portion. Later rupture is seen if the ovum implants within the interstitial portion. Rupture is usually spontaneous but can also follow trauma such as that associated with bimanual pelvic examination or coitus.
After implantation, differences in ectopic pregnancy development explain the typically divergent clinical paths between acute and chronic ectopic pregnancies. Acute ectopic pregnancies are those with a high serum β-hCG level at presentation. These high β-hCG levels correlate with the depth of trophoblastic invasion into the tubal wall. Greater invasion promotes concomitant severe ischemic changes and tubal wall rupture (Erol, 2015). Rapid pregnancy growth leads to an immediate diagnosis from painful tubal distention or from rupture. Indeed, these carry a higher risk of tubal rupture compared with chronic ectopic pregnancies (Barnhart, 2003c).
With chronic ectopic pregnancy, minor repeated ruptures or tubal abortion incites an inflammatory response that leads to formation of a pelvic mass. Its abnormal trophoblasts die early. Thus, negative or lower, static serum β-hCG levels are found. Chronic ectopic pregnancies typically rupture late, if at all, but commonly form a complex pelvic mass. In these cases, it often is the mass, rather than pain or bleeding, that prompts diagnostic surgery (Cole, 1982; Uğur, 1996).
CLINICAL MANIFESTATIONS
The classic symptom triad of ectopic pregnancy is amenorrhea followed by vaginal bleeding and ipsilateral abdominal pain. However, as women seek care earlier, the ability to diagnose ectopic pregnancy before rupture—even before the onset of symptoms—is not unusual. Of other symptoms, banal pregnancy discomforts such as breast tenderness, nausea, and urinary frequency may accompany more ominous findings. These include shoulder pain worsened by inspiration, which is caused by phrenic nerve irritation from subdiaphragmatic blood, or vasomotor disturbances such as vertigo and syncope from hemorrhagic hypovolemia.
Of physical findings, some women have orthostatic findings from hypovolemia. Birkhahn and associates (2003) employed the shock index to evaluate the severity of ruptured ectopic pregnancy. This index is the heart rate divided by systolic blood pressure and can assess trauma patients for hypovolemic or septic shock. The normal range lies between 0.5 and 0.7 for nonpregnant patients. A shock index >0.85 and a systolic blood pressure <110 mm Hg are highly suggestive of a potentially life-threatening gynecologic emergency, such as a ruptured ectopic pregnancy (Birkhahn, 2003; Polena, 2015). Despite these findings of advanced hypovolemia, normal vital signs are unreliable to exclude earlier stages of tubal rupture.
Abdominal and pelvic findings may also be notoriously scarce in many women before tubal rupture. With rupture, however, nearly three fourths will have marked tenderness on both abdominal and pelvic examination, and pain is aggravated with cervical manipulation. A pelvic mass, including fullness posterolateral to the uterus, can be palpated in approximately 20 percent of women. Initially, an ectopic pregnancy may feel soft and elastic, whereas extensive intraluminal hemorrhage produces a firmer consistency. Many times, discomfort precludes palpation of the mass, and limiting examinations may help avert iatrogenic rupture.
DIAGNOSIS
Symptoms of ectopic pregnancy can mimic multiple entities (Table 7-2). Early pregnancy complications such as threatened or missed abortion or hemorrhagic corpus luteum cyst may be difficult to differentiate. Moreover, approximately 20 percent of women with normal pregnancies have early bleeding. Several disorders not related to pregnancy can also mimic ectopic pregnancy. In general, a positive test for β-hCG usually excludes these other diagnoses. However, these conditions may exist concurrently with pregnancy—either intrauterine or ectopic. Transvaginal sonography and serial serum β-hCG measurements are the most valuable diagnostic aids to confirm clinical suspicions of an ectopic pregnancy. Additionally, because ectopic pregnancy can lead to significant bleeding, a hemogram is an additional fast and effective initial screen.
| Cause | Abdominal Location | Characteristics | Associated Findings |
| Pregnancy | |||
| Abortion Ectopic | Midline or generalized Unilateral or generalized | Crampy, episodic Sharp or aching, continuous | (+) UCG; vaginal bleeding (+) UCG; vaginal bleeding |
| Uterus and Cervix | |||
| Endomyometritis Endometriosis Degenerating myoma | Lower, midline Lower, midline Lower, midline | Dull aching Cyclic, aching Dull aching or sharp | Vaginal discharge, fever Possible adnexal mass Irregular, enlarged uterus |
| Adnexal Disease | |||
| Salpingitis Tuboovarian abscess Corpus luteum cyst Adnexal torsion | Unilateral or bilateral Unilateral or bilateral Lower, unilateral Lower, unilateral | Severe Dull aching or sharp Acute onset, sharp Acute onset, sharp, continuous or episodic | Moderate to high fever High fever; adnexal mass (+/−) UCG Adnexal mass |
| Other | |||
| Appendicitis Diverticulitis Cystitis Renal calculi | Periumbilical or right lower lower Midline, suprapubic Flank, radiating downward | Sharp or aching, continuous Dull aching Acute, spasms Severe, episodic | Anorexia, nausea, vomiting Fever Dysuria, frequency Hematuria |
Human chorionic gonadotropin is a glycoprotein produced by syncytiotrophoblast and can be detected in serum as early as 8 days after the luteinizing hormone (LH) surge. In normal pregnancies, serum β-hCG levels rise in a log-linear fashion until 60 or 80 days after the last menses, at which time values plateau at approximately 100,000 IU/L. Given an interassay variability of 5 to 10 percent, interpretation of serial values is more reliable when performed by the same laboratory.
With a robust intrauterine pregnancy (IUP), serum β-hCG levels should increase at least 53 to 66 percent every 48 hours (Barnhart, 2004; Kadar, 1982). Seeber and associates (2006) used an even more conservative 35-percent rise after 48-hours. Past this 48 hours, allowing time for additional data may better determine the location and viability of the pregnancy. But, this is weighed against the increased chance of ectopic pregnancy rupture during these extra diagnostic days. In hemodynamically stable women, adding a third serum β-hCG level on day 4 or 7 could correct the diagnosis of a pregnancy of unknown location in an additional 7 to 13 percent of patients (Zee, 2013). Nevertheless, inadequately rising serum β-hCG levels indicate only a dying pregnancy, not its location.
Many women present with an unsure last menstrual period, and an educated guess of gestational age is made. In these cases, correlation between the serum β-hCG concentration and transvaginal sonography findings becomes especially important.
Serum progesterone concentration is used by some to aid ectopic pregnancy diagnosis when serum β-hCG levels and sonographic findings are inconclusive (Stovall, 1992). Serum progesterone concentration varies minimally between 5 and 10 weeks’ gestation, thus a single value is sufficient. Mol and coworkers (1998) performed a metaanalysis of 22 studies to assess the accuracy of a single serum progesterone level to differentiate ectopic from uterine pregnancy. They found that results were most accurate when approached from the viewpoint of healthy versus dying pregnancy. With serum progesterone levels <5 ng/mL, a dying pregnancy was detected with near perfect specificity and with a sensitivity of 60 percent. Conversely, values of >20 ng/mL had a sensitivity of 95 percent with specificity approximating 40 percent to identify a healthy pregnancy. Ultimately, serum progesterone levels can be used to buttress a clinical impression, but again they cannot reliably differentiate between an ectopic and intrauterine pregnancy (Guha, 2014).
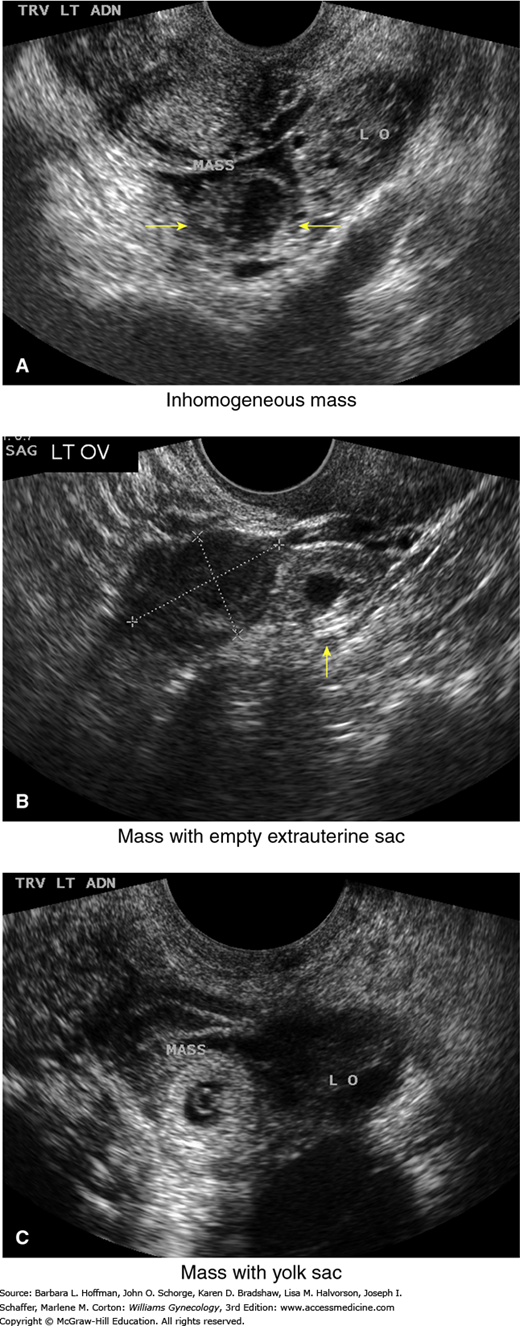
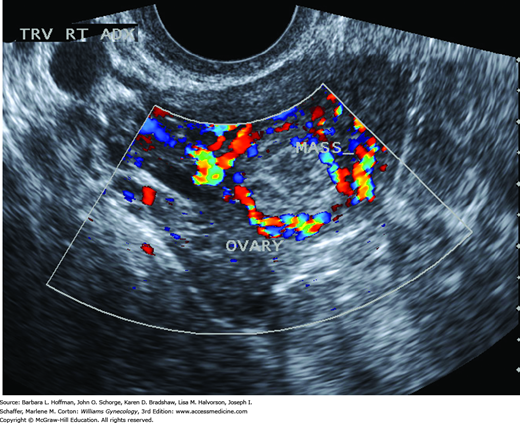
High-resolution sonography has revolutionized the clinical management of women with a suspected ectopic pregnancy. With transvaginal sonography (TVS), a gestational sac is usually visible between 4½ and 5 weeks, the yolk sac appears between 5 and 6 weeks, and a fetal pole with cardiac activity is first detected at 5½ to 6 weeks. With transabdominal sonography, these structures are visualized slightly later. The sonographic diagnosis of ectopic pregnancy rests on visualization of an adnexal mass separate from the ovary (Fig. 7-3).
FIGURE 7-3
Transvaginal sonographic findings with various ectopic pregnancies. For sonographic diagnosis, an ectopic mass should be seen in the adnexa separate from the ovary and may be seen: (A) as an inhomogeneous adnexal mass (yellow arrows), (B) as an empty extrauterine sac with a hyperechoic ring (arrow), or (C) as a yolk sac and/or fetal pole with or without cardiac activity within an extrauterine sac. LO = left ovary. (Used with permission from Dr. Elysia Moschos.)
When the last menstrual period is unknown, serum β-hCG testing is used to define expected sonographic findings. Each institution must define a β-hCG discriminatory value for TVS, that is, the lower limit at which an examiner can reliably visualize an IUP. At most institutions, this value is a concentration between 1500 and 2000 IU/L. Accurate diagnosis by sonography is three times more likely if the initial β-hCG level is above this value. Connolly and colleagues (2013) reported evidence to suggest an even higher threshold. They noted that with live IUPs, a gestational sac was seen 99 percent of the time with a discriminatory level of 3510 IU/L. Even with β-hCG levels above the chosen discriminatory value, technical challenges such as leiomyomas, adenomyosis, multifetal gestation, or IUD can hinder the ability to accurately diagnose an intrauterine gestation (Gurel, 2007; Ko, 2014).
When β-hCG levels are above the set discriminatory value, the absence of an IUP may suggest an abnormal pregnancy. The abnormality may be an ectopic pregnancy, an incomplete abortion, or a resolving completed abortion. For example, despite total passage of products of conception with complete abortion, β-hCG testing may still be positive while original β-hCG is metabolized and cleared. Conversely, when β-hCG values lie below the discriminatory value, sonographic findings are not diagnostic in nearly two thirds of cases (Barnhart, 1999).
In an attempt to unify the language used with sonographic evaluation of early pregnancies, a consensus statement was drafted with five categories: (1) definitive ectopic pregnancy (extrauterine gestational sac with yolk sac and/or embryo), (2) probable ectopic pregnancy (inhomogeneous adnexal mass or extrauterine sac-like structure), (3) probable IUP (intrauterine echogenic sac), (4) definite IUP (intrauterine gestational sac with yolk sac and/or embryo), and (5) pregnancy of unknown location (PUL) (lacking signs of either ectopic pregnancy or IUP) (Barnhart, 2011).
Systematic sonographic evaluation is critical to establish the correct diagnosis. Most begin with the endometrial cavity. In pregnancies conceived spontaneously, identification of an IUP effectively excludes the possibility of ectopic implantation. When ART is employed, however, careful examination of the tube and ovary is performed even with an intrauterine pregnancy because heterotopic pregnancy rates may be as high as 1 per 100 (Tal, 1996). An intracavitary fluid collection caused by bleeding from the decidua can create a pseudogestational sac, or pseudosac. As shown in Figure 7-4, this one-layer collection lies typically in the midline of the uterine cavity. In contrast, a normal gestational sac is eccentrically located (Dashefsky, 1988). Another intracavitary finding is a trilaminar endometrial pattern, which represents two adjacent proliferative-phase endometrial layers (Fig. 2-16) (Lavie, 1996). For the diagnosis of ectopic pregnancy, this finding’s specificity is 94 percent but with a sensitivity of only 38 percent (Hammoud, 2005). Endometrial stripe thickness has not been well correlated with ectopic pregnancies. However, Moschos and Twickler (2008b) determined that in PULs, none that ultimately proved to be normal IUPs had a stripe thickness <8 mm.
The fallopian tubes and ovaries are also inspected. Visualization of an extrauterine yolk sac or embryo clearly confirms an ectopic pregnancy, although such findings are less commonly seen (Paul, 2000). In some cases, a halo or tubal ring that surrounds an anechoic sac can be seen. According to Burry and associates (1993), this has a positive-predictive value of 92 percent and a sensitivity of 95 percent. Alternatively, an inhomogeneous complex adnexal mass is usually caused by hemorrhage within the ectopic sac or by an ectopic pregnancy that has ruptured into the tube. Overall, approximately 60 percent of ectopic pregnancies are seen as an inhomogeneous mass adjacent to the ovary; 20 percent appear as a hyperechoic ring; and 13 percent have an obvious gestational sac with a fetal pole (Condous, 2005). Brown and associates (1994) conducted a metaanalysis of 10 studies to ascertain the best transvaginal sonographic criteria to diagnose ectopic pregnancy. They reported that the finding of any adnexal mass, other than a simple ovarian cyst, was the most accurate. With this, they found a sensitivity of 84 percent, specificity of 99 percent, positive-predictive value of 96 percent, and negative-predictive value of 95 percent. However, not all adnexal masses represent an ectopic pregnancy, and integration of sonographic findings with other clinical information is necessary.
Differentiating an ectopic pregnancy from a corpus luteum cyst can be challenging. However, Swire and coworkers (2004) observed that the corpus luteum wall is less echogenic compared with both a tubal ring and the endometrium. They found that a spongelike, lacelike, or reticular pattern seen within the cyst is classic for hemorrhage (Fig. 9-16). Moreover, a corpus luteum is found within the parenchyma of an ovary, but a markedly asymmetric appearing ovary should raise suspicion of an ectopic pregnancy (Gurel, 2007). With transvaginal color Doppler imaging, placental blood flow within the periphery of the ectopic pregnancy—the ring of fire—can be seen (Fig. 7-5). Although this finding can aid ectopic pregnancy diagnosis, a ring of fire also can be seen with a corpus luteum of pregnancy (Pellerito, 1992). Pulsed-color Doppler sonographic measurement of resistance indices has poor sensitivity and limits its utility (Atri, 2003). Finally, to help characterize a suspicious mass, an examiner can gently palpate an adnexum that is placed between the vaginal probe and the examiner’s abdominal hand during real-time scanning. A mass that moves separately from the ovary suggests a tubal pregnancy, whereas a mass that moves synchronously more likely represents a corpus luteum cyst (Levine, 2007).
During sonographic evaluation of the pelvis, TVS can detect as little as 50 mL of free peritoneal fluid in the cul-de-sac of Douglas. This may be intraabdominal bleeding or physiologic peritoneal fluid. A large volume of fluid or fluid that is echogenic is more worrisome for hemoperitoneum. In addition, transabdominal right-upper-quadrant sonographic imaging helps assess the extent of hemoperitoneum. Blood in the paracolic gutters and Morison pouch indicates significant hemorrhage. Specifically, free fluid in Morison pouch typically is not seen until a hemoperitoneum reaches 400 to 700 mL (Branney, 1995; Rodgerson, 2001). Detection of peritoneal fluid in conjunction with an adnexal mass is highly predictive of ectopic pregnancy (Nyberg, 1991). That said, despite technologic advances, the absence of suggestive findings does not exclude ectopic pregnancy.
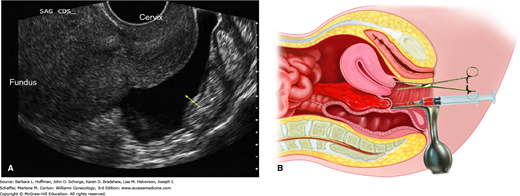
With a 16- to 18-gauge spinal needle, the cul-de-sac of Douglas may be entered through the posterior vaginal fornix (Fig. 7-6). The aspirate characteristics, in conjunction with clinical findings, may help clarify the diagnosis. Normal-appearing peritoneal fluid is designated as a negative test. If fragments of an old clot or nonclotting blood are found in the aspirate when placed into a dry, clean test tube, then hemoperitoneum is diagnosed. If the aspirated blood clots after it is withdrawn, this may signify active intraperitoneal bleeding or puncture of an adjacent vessel. If fluid cannot be aspirated, the test can only be interpreted as unsatisfactory. Purulent fluid suggests an infection-related cause such as salpingitis or appendicitis. Feculent material may originate from a perforated colon or an inadvertent puncture of the rectosigmoid colon during culdocentesis.
FIGURE 7-6
A. Transvaginal sonography of a fluid collection (arrow) in the cul-de-sac of Douglas. (Used with permission from Dr. Elysia Moschos.) B. Culdocentesis. With a 16- to 18-gauge spinal needle attached to a syringe, the cul-de-sac of Douglas is entered through the posterior vaginal fornix as upward traction is applied to the cervix with a tenaculum.
Several studies have challenged the usefulness of this bedside test, and culdocentesis has been largely replaced by TVS (Glezerman, 1992; Vermesh, 1990). Sonography with findings of echogenic fluid to establish hemoperitoneum is more sensitive and specific than culdocentesis—100 and 100 percent versus 66 and 80 percent, respectively. Also, for most women, sonography is better tolerated.
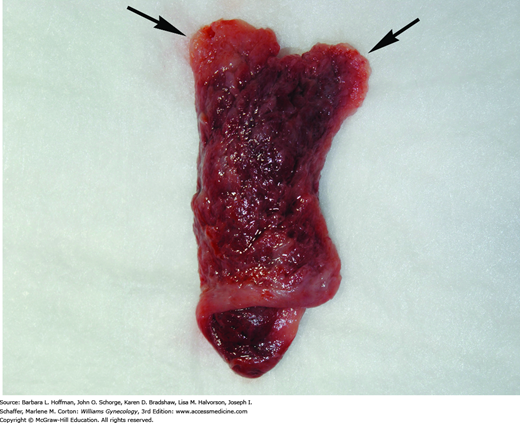
Several endometrial changes are associated with ectopic pregnancy. These include decidua found in 42 percent of samples, secretory endometrium in 22 percent, and proliferative endometrium in 12 percent, all with an absence of trophoblasts (Lopez, 1994). Decidua is endometrium that is hormonally prepared for pregnancy, and the degree to which the endometrium is converted with ectopic pregnancy is variable. Thus, in addition to bleeding, women with ectopic tubal pregnancy may pass a decidual cast, which is the entire sloughed endometrium that reflects the form of the endometrial cavity (Fig. 7-7). Decidual sloughing may also occur with IUP abortion. Thus, tissue is carefully evaluated visually and then histologically for evidence of a conceptus. If no clear gestational sac is visually seen or if no villi are identified histologically within the cast, then the possibility of ectopic pregnancy must still be entertained.
Before methotrexate treatment is given for ectopic pregnancy treatment, many recommend that the absence of intrauterine trophoblastic tissue be confirmed by curettage (Barnhart, 2002; Chung, 2011; Shaunik, 2011). The presumptive diagnosis of ectopic pregnancy is inaccurate in nearly 40 percent of cases without histologic exclusion of a spontaneous pregnancy loss. Nevertheless, the need, method, and risks of endometrial sampling must carefully be weighed against the limited risks of methotrexate.
Endometrial biopsy with a Pipelle catheter was studied as an alternative to curettage and found inferior. The sensitivity of obtaining chorionic villi ranged from 30 to 63 percent (Barnhart, 2003b; Ries, 2000). By comparison, frozen section of curettage fragments to identify products of conception is accurate in more than 90 percent of cases (Barak, 2005; Li, 2014b; Spandorfer, 1996).
Confirmation by diagnostic laparoscopy remains the gold standard for ectopic pregnancy diagnosis (Fig. 7-8). That said, with sensitive diagnostic modalities available, ectopic pregnancy can typically be diagnosed prior to surgery, and use of an evidence-based algorithm can assist. After appropriate clinical evaluation, all reproductive-aged women with any suspicion of pregnancy are tested using a sensitive urine β-hCG assay. Following positive testing, if an IUP is not confirmed by sonography, if no signs of acute intraabdominal hemorrhage are present, and if an ectopic gestation is suspected, then an evaluation such as the one depicted in Figure 7-9 may be used. Gracia and Barnhart (2001) performed a decision analysis of six diagnostic strategies to evaluate which sequence of tests was most efficient in missing the fewest ectopic pregnancies and interrupting the fewest IUPs. They found the best strategy was to include TVS for all women with first-trimester pain or bleeding. If findings are not diagnostic, then serial serum β-hCG levels are measured. Using this strategy, only 1 percent of all potential IUPs were interrupted; no ectopic pregnancies were missed; and the average time to diagnosis was 1.46 days.